Abstract
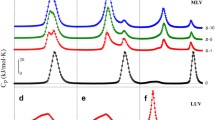
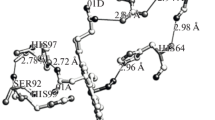
Interaction mode of an inhalation anesthetic halothane with water-soluble globular proteins, myoglobin (Mgb) and lysozyme (Lys), was studied by differential scanning calorimetry (DSC) and viscometry, and the results were compared with those of bovine serum albumin (BSA). The anesthetic sensitivity was markedly different among the proteins: Mgb was destabilized, Lys was slightly destabilized, and BSA was conversely stabilized. Further, the interaction mode was quite different from those of specific binders for the proteins. The anesthetic sensitivity was highly correlated with the hydrophilicity on the protein surface (Mgb < Lys < BSA) and the rigidity of the protein structure (BSA ≈ Mgb < Lys). We showed that the anesthetic sensitivity among globular proteins can be roughly classified into four categories, and proteins with small hydrophilicity and soft structure are suitable as model proteins of anesthesia. By contrast, the binding of the specific binders was characterized by the lower effective concentrations. The molar ratio of the binder to the protein at the effective concentration was well consistent with the binding number determined from the X-ray structural analysis. Moreover, the interaction mode of the binder was not necessarily in accord with the mode expected from the change in the protein structure. Considering the above facts, we can systematically interpret the effect of an anesthetic on globular proteins by four factors: (1) hydrophobicity of an anesthetic, (2) hydrophilicity of a protein surface, (3) rigidity of a protein structure, and (4) molar ratio of an anesthetic to a protein at the effective concentration.










Similar content being viewed by others
References
Meyer HH (1899) Welche eigenschaft der anasthetica bedingt inre Narkotische wirkung? Arch Exp Pathol Pharmakol 42:109–118
Overton CE (1901) Studien über die Narkose zugleich ein Beitrag zur allgemeinen Pharmakologie. Gustav Fischer, Jena, Switzerland
Franks NP, Lieb WR (1978) Where do general anesthetics act? Nature 274:339–342
Franks NP, Lieb WR (1982) Molecular mechanisms of general anaesthesia. Nature 300:487–493
Franks NP, Lieb WR (1994) Molecular and cellular mechanisms of general anaesthesia. Nature 367:607–614
Cantor RS (1998) The lateral pressure profile in membranes: a physical mechanism of general anesthesia. Toxicol Lett 100–101:451–458
Okamura E, Nakahara M (1999) NMR study directly determining drug delivery sites in phospholipid bilayer membranes. J Phys Chem B 103:3505–3509
Koubi L, Tarek M, Klein ML, Scharf D (2000) Distribution of halothane in a dipalmitoylphosphatidylcholine bilayer from molecular dynamics calculations. Biophys J 78:800–811
Heimburg T, Jackson AD (2007) The thermodynamics of general anesthesia. Biophys J 92:3159–3165
Yamamoto Y, Ando T, Takayama M, Egami T, Ohtsu Y, Sakurai A, Yoshinda T, Taga K, Kamaya H, Ueda I (2008) Interaction between phospholipid monolayer and volatile anesthetics using quartz crystal oscillator methods. Colloids Surf A: Physicochem Eng Aspect 317:568–575
Schoenborn BP, Watson HC, Kendrew JC (1965) Binding of xenon to sperm whale myoglobin. Nature 207:28–30
Schoenborn BP (1967) Binding of cyclopropane to sperm whale myoglobin. Nature 214:1120–1122
Dong A, Huang P, Zhao X-J, Sampath V, Caughey WS (1994) Characterization of sites occupied by the anesthetic nitrous oxide within proteins by infrared spectroscopy. J Biol Chem 269:23911–23917
Eckenhoff RG, Tanner JW (1998) Differential halothane binding and effects on serum albumin and myoglobin. Biophys J 75:477–483
Tanner JW, Johansson JS, Liebman PA, Eckenhoff RG (2001) Predictability of weak binding from X-ray crystallography: inhaled anesthetics and myoglobin. Biochemistry 40:5075–5080
Eckenhoff RG, Pidikiti R, Reddy KS (2001) Anesthetic stabilization of protein intermediates: myoglobin and halothane. Biochemistry 40:10819–10824
Streiff JH, Juranic NO, Macura SI, Warner DO, Jones KA, Perkins WJ (2004) Saturation transfer difference nuclear magnetic resonance spectroscopy as a method for screening proteins for anesthetic binding. Mol Pharmacol 66:929–935
Ueda I, Suzuki A (1998) Irreversible phase transition of firefly luciferase: contrasting effects of volatile anesthetics and myristic acid. Biochim Biophys Acta 1380:313–319
Matsuki H, Suzuki A, Kamaya H, Ueda I (1999) Specific and non-specific binding of long-chain fatty acids to firefly luciferase: cutoff at octanoate. Biochim Biophys Acta 1426:143–150
Ueda I, Matsuki H, Kamaya H, Krishna PR (1999) Does pressure antagonize anesthesia? Opposite effect on specific and nonspecific inhibitors of firefly luciferase. Biophys J 78:483–488
Eckenhoff RG, Tanner JW, Liebman PA (2001) Cooperative binding of inhaled anesthetics and ATP to firefly luciferase. Protein Struct Funct Genet 42:436–441
Takehara K, Kamaya H, Ueda I (2005) Inhibition of firefly luciferase by alkane analogues. Biochim Biophys Acta 1721:124–129
Szarecka A, Xu Y, Tang P (2007) Dynamics of firefly luciferase inhibition by general anesthetics: gaussian and anisotropic network analyses. Biophys J 93:1895–1905
Isogai H, Seto T, Nosaka S (2005) Validation of hypothesis of pressure reversal mechanisms: “anesthetic binds to site, and squeezed out by pressure”. Int Cong Ser 1283:318–319
Imai T, Isogai H, Seto T, Kovalenko A, Hirata F (2006) Theoretical study of volume changes accompanying xenon-lysozyme binding: implications for the molecular mechanism of pressure reversal of anesthesia. J Phys Chem B 110:12149–12154
Dubois BW, Cherian SF, Evers AS (1993) Volatile anesthetics compete for common binding sites on bovine serum albumin: a 19F-NMR study. Proc Natl Acad Sci USA 90:6478–6482
Johansson JS, Eckenhoff RG, Dutton PL (1995) Binding of halothane to serum albumin demonstrated using tryptophan fluorescence. Anesthesiology 83:316–324
Eckenhoff RG (1996) Amino acid resolution of halothane binding sites in serum albumin. J Biol Chem 271:15521–15526
Yoshida T, Tanaka M, Mori Y, Ueda I (1997) Negative entropy of halothane binding to protein: 19F-NMR with a novel cell. Biochim Biophys Acta 1334:117–122
Tanner JW, Eckenhoff RG, Liebman PA (1999) Halothane, an inhalational anesthetic agent, increases folding stability of serum albumin. Biochim Biophys Acta 1430:46–56
Yamanaka M, Kaneshina S, Kamaya H, Ueda I (2001) Volumetric study on the protein-anesthetic binding. Colloid Surf B Biointerf 22:23–29
Sawas AH, Pentyala SN, Rebecchi MJ (2004) Binding of volatile anesthetics to serum albumin: measurements of enthalpy and solvent contributions. Biochemistry 43:12675–12685
Ueda I, Yamanaka M (1997) Titration calorimetry of anesthetic–protein interaction: negative enthalpy of binding and anesthetic potency. Biophys J 72:1812–1817
Tanner JW, Liebman PA, Eckenhoff RG (1998) Volatile anesthetics alter protein stability. Toxicol Lett 100–101:387–391
Matsuki H, Komatsu U, Nishimoto M, Kaneshina S, Ogli K (2005) Comparative study of specific and non-specific interactions between bio-macromolecules and ligands. Int Cong Ser 1283:207–210
Nishimoto M, Hata T, Goto M, Tamai N, Kaneshina S, Matsuki H, Ueda I (2009) Interaction modes of long-chain fatty acids in dipalmitoylphosphatidylcholine bilayer membrane: contrast to mode of inhalation anesthetics. Chem Phys Lipids 158:71–80
Nishimoto M, Komatsu U, Tamai N, Yamanaka M, Kaneshina S, Ogli K, Matsuki H (2009) A comparative study on specific and nonspecific interactions in bovine serum albumin: thermal and volume effects of halothane and palmitic acid. Colloid Polym Sci 287:979–989
Nishimoto M, Morimitsu T, Tamai N, Kaneshina S, Nagamune H, Matsuki H (2010) Inhibition of anti-fluorescent probe monoclonal antibody by long-chain amphiphiles. Colloids Surf B Biointerf 75:80–87
Ogli K, Komatsu U, Matsuki H, Kaneshina S (2005) Effect of halotane on the viscosity of aqueous bovine serum albumin solutions. Anesth Resusc Jpn 41:39–42
Privalov PL, Khechinashvili NN (1974) A thermodynamic approach to the problem of stabilization of globular protein structure: a calorimetric study. J Mol Biol 86:665–684
Privalov PL, Tiktopulo EI, Venyaminov SYu, Griko YuV, Makhatadze GI, Khechinashvili NN (1989) Heat capacity and conformation of proteins in the denatured state. J Mol Biol 205:737–750
Blanco E, Ruso JM, Sabin J, Prieto G, Sarmiento F (2007) Thermal stability of lysozyme and myoglobin in the presence of anionic surfactants. J Therm Anal Cal 87:211–215
Torreggiani A, Di Foggia M, Manco I, De Maio A, Markarian SA, Bonora S (2008) Effect of sulfoxides on the thermal denaturation of hen lysozyme: a calorimetric and Raman study. J Mol Struct 891:115–122
Kamiyama T, Liu HL, Kimura T (2009) Preferential solvation of lysozyme by dimethyl sulfoxide in binary solutions of water and dimethyl sulfoxide. J Therm Anal Cal 95:353–359
Stigter D, Alonso DOV, Dill KA (1991) Protein stability: electrostatics and compact denatured states. Proc Natl Acad Sci USA 88:4176–4180
Chipman DM, Sharon N (1969) Mechanism of lysozyme action. Lysozyme is the first enzyme for which the relation between structure and function has become clear. Science 165:454–465
Von Dreele RB (2001) Binding of N-acetylglucosamine to chicken egg lysozyme: a powder diffraction study. Acta Crystallogr D Biol Crstallogr 57:1836–1842
Von Dreele RB (2005) Binding of N-acetylglucosamine oligosaccharides to hen egg-white lysozyme: a powder diffraction study. Acta Crystallogr D Biol Crstallogr 61:22–32
Xie G, Timasheff SN (1997) The thermodynamic mechanism of protein stabilization by trehalose. Biophys Chem 64:25–43
Hédoux A, Willart J-F, Ionov R, Affouard F, Guinet Y, Paccou L, Lerbret A, Descamps M (2006) Analysis of sugar bioprotective mechanisms on the thermal denaturation of lysozyme from Raman scattering and differential scanning calorimetry investigations. J Phys Chem B 110:22886–22893
Gopal S, Ahluwalia JC (1995) Differential scanning calorimetric studies on binding of N-acetyl-d-glucosamine to lysozyme. Biophys Chem 54:119–125
Monkos K (1997) Concentration and temperature dependence of viscosity in lysozyme aqueous solutions. Biochim Biophys Acta 1339:304–310
Zhou H-X (1995) Calculation of translational friction and intrinsic viscosity. II. Application to globular proteins. Biophys J 69:2298–2303
Kyte J, Doolittle RF (1982) A simple method for displaying the hydropathic character of a protein. J Mol Biol 157:105–132
Nozaki Y, Tanford C (1971) The solubility of amino acids and two glycine peptides in aqueous ethanol and dioxane solutions: establishment of a hydrophobicity scale. J Biol Chem 246:2211–2217
Bigelow CC (1967) On the average hydrophobicity of proteins and the relation between it and protein structure. J Theor Biol 16:187–211
Seto T, Mashimo T, Yoshiya I, Kaneshiro M, Taniguchi Y (1998) Solubility and preparation of volatile anesthetic solution. J Anesth 12:41–42
Ueda I (1989) Anesthesia: an interfacial phenomenon. Colloids Surf 38:37–48
Ben-Naim A (1980) Temperature and pressure dependence of the hydrophobic interactions. Hydrophobic interactions, Plenum Press, New York, In, pp 185–258
Mukae K, Sakurai M, Sawamura S, Makino K, Kim SW, Ueda I, Shirahama K (1993) Swelling of poly(N-isopropylacrylamide) gels in water–alcohol (C1–C4) mixed solvents. J Phys Chem 97:737–741
Gekko K, Noguchi H (1979) Compressibility of globular proteins in water at 25°C. J Phys Chem 83:2706–2714
Gekko K, Hasegawa Y (1986) Compressibility–structure relationship of globular proteins. Biochemistry 25:6563–6571
Gekko K, Hasegawa Y (1989) Effect of temperature on the compressibility of native globular proteins. J Phys Chem 93:426–429
Takekiyo T, Imai T, Kato M, Taniguchi Y (2006) Understanding high pressure stability of helical conformation of oligopeptides and helix bundle proten, high pressure FT-IR and RISM theoretical studies. Biochim Biophys Acta 1764:355–363
Takekiyo T, Takeda N, Isogai Y, Kato M, Taniguchi Y (2007) Pressure stability of the α-helix structure in a de novo designed protein (α-I-α)2 studied by FTIR spectroscopy. Biopolymers 85:185–188
Imamura H, Kato M (2009) Effect of pressure on helix-coil transition of an alanine-based peptide: an FTIR study. Protein Struct Funct Bioinforma 75:911–918
Yamato T, Higo J, Seno Y, Go N (1993) Conformational deformation in deoxymyoglobin by hydrostatic pressure. Protein Struct Funct Bioinforma 16:327–340
Bhattacharya AA, Grüne T, Curry S (2000) Crystallography analysis reveals common modes of binding of medium and long-chain fatty acids to human serum albumin. J Mol Biol 303:721–732
Ghuman J, Zunszain PA, Petitpas I, Bhattacharya AA, Otagiri M, Curry S (2005) Structural basis of the drug-binding specificity of human serum albumin. J Mol Biol 353:38–52
Gekko K, Yamagami K (1998) Compressibility and volume changes of lysozyme due to inhibitor binding. Chem Lett 27:839–840
Franks NP, Jenkins A, Conti E, Lieb WR, Brick P (1998) Structural basis for the inhibition of firefly luciferase by a general anesthetic. Biophys J 75:2205–2211
Bhattacharya AA, Curry S, Franks NP (2000) Binding of the general anesthetics propofol and halothane to human serum albumin. J Biol Chem 275:38731–38738
Reed RG (1986) Location of long chain fatty acid-binding sites of bovine serum albumin by affinity labeling. J Biol Chem 261:15619–15624
Cistola DP, Small DM, Hamilton JA (1987) Location of long chain fatty acid-binding sites of bovine serum albumin by affinity labeling. J Biol Chem 262:10971–10979
Acknowledgement
The authors all thank the late Professor Issaku Ueda at University of Utah for his encouragement and suggestions for researches of the molecular mechanism of anesthesia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nishimoto, M., Komatsu, U., Tamai, N. et al. Intrinsic interaction mode of an inhalation anesthetic with globular proteins: a comparative study on ligand recognition. Colloid Polym Sci 289, 1785–1797 (2011). https://doi.org/10.1007/s00396-011-2491-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-011-2491-z