Abstract
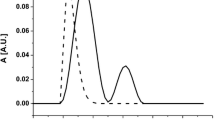
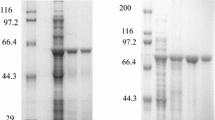
The thermal stabilization and refolding of horseradish peroxidase (HRP) upon heating were investigated using an artificial molecular chaperone consisting of cholesterol-bearing pullulan (CHP) nanogels. The CHP nanogels inhibited the aggregation of HRP under heating by complexation with the denatured HRP. The enzyme activity of HRP complexed with CHP nanogels was not detected. However, the enzyme activity recovered up to 80% of native HRP after the addition of cyclodextrin (CD) to the complex. The dissociation of CHP nanogels was induced by the formation of an inclusion complex of cholesterol groups of CHP with CD. The enzyme activity of HRP was only significantly recovered by the addition of β-CD or its derivatives. Natural molecular chaperones, such as GroEL/ES, trap, fold, and release the nonnative proteins by changing the hydrophobicity of the specific sites of the molecular chaperone that interact with the nonnative protein. The functional mechanism of the nanogel chaperon system is similar to that of natural molecular chaperones. The nanogel chaperone system is a useful tool to aid the refolding and thermal stabilization of unstable proteins for post-genome research, and in medical and biological applications.






Similar content being viewed by others
References
Stuart MA, Huck WT, Genzer J, Müller M, Ober C, Stamm M, Sukhorukov GB, Szleifer I, Tsukruk VV, Urban M, Winnik F, Zauscher S, Luzinov I, Minko S (2010) Emerg applications stimuli responsive polymer materials. Nat Mater 9:101–113
Du FS, Wang Y, Zhang R, Li ZC (2010) Intelligent nucleic acid delivery systems based on stimuli-responsive polymers. Soft Matter 6:835–848
Asoh TA, Akashi M (2009) Development of high-performance stimuli-responsive systems. In: Stein DB (ed) Handbook of hydrogels: properties, preparation and applications (chemical engineering methods and technology series). Nova Science Publishers, Hauppauge, pp 633–648
Li MH, Keller P (2009) Stimuli-responsive polymer vesicles. Soft Matter 5:927–937
Xia F, Zhu Y, Feng L, Jiang L (2009) Smart responsive surfaces switching reversibly between super-hydrophobicity and super-hydrophilicity. Soft Matter 5:275–281
Cole MA, Voelcker NH, Thissen H, Griesser HJ (2009) Stimuli-responsive interfaces and systems for the control of protein-surface and cell-surface interactions. Biomaterials 30:1827–1850
Kloxin M, Kloxin CJ, Bowman CN, Anseth KS (2010) Mechanical properties of cellularly responsive hydrogels and their experimental determination. Adv Mater 22:3484–3494
Vinogradov SV, Batrakova EV, Kabanov AV (1999) Poly-(ethylene glycol)-polyethyleneimine NanoGel particles: novel drug delivery systems for antisense oligonucleotides. Colloids Surf B 16:291–304
Oh JK, Drumright R, Siegwart DJ, Matyjaszewski K (2008) The development of microgels/nanogels for drug delivery applications. Prog Polym Sci 33:448–477
Sasaki Y, Akiyoshi K (2010) Nanogel engineering for new nanobiomaterials: from chaperoning engineering to biomedical applications. The Chemical Record 10:366–376
Reamdonck K, Demeester J, De Smedt S (2009) Advanced nanogel engineering for drug delivery. Soft Matter 5:707–715
Roy D, Cambre JN, Sumerlin BS (2010) Future perspectives and recent advances in stimuli-responsive materials. Prog Polym Sci 35:278–301
Ju XJ, Xie R, Yang L, Chu LY (2009) Biodegradable 'intelligent' materials in response to chemical stimuli for biomedical applications. Expert Opin Ther Pat 19:683–696
Akiyoshi K, Deguchi S, Moriguchi N, Yamaguchi S, Sunamoto J (1993) Self-aggregates of hydrophobized polysaccharides in water-formation and characteristics of nanoparticles. Macromolecules 26:3062–3068
Akiyoshi K, Deguchi S, Tajima H, Kishikawa T, Sunamoto J (1997) Microscopic structure and thermoresponsiveness of a hydrogel nanoparticle by self-assembly of a hydrophobized polysaccharide. Macromolecules 30:857–861
Nishikawa T, Akiyoshi K, Sunamoto J (1996) Macromolecular complexation between bovine serum albumin and self-assembled hydrogel nanoparticle of hydrophobized polysaccharides. J Am Chem Soc 118:6110–6115
Ikuta Y, Katayama N, Wang L, Okugawa T, Takahashi Y, Schmitt M, Gu X, Watanabe M, Akiyoshi K, Nakamura H, Kuribayashi K, Sunamoto J, Shiku H (2002) Presentation of a major histocompatibility complex class 1-binding peptide by monocyte-derived dendritic cells incorporating hydrophobized polysaccharide-truncated HER2 protein complex: implications for a polyvalent immuno-cell therapy. Blood 99:3717–3724
Nochi T, Yuki Y, Takahashi H, Sawada SI, Mejima M, Kohda T, Harada N, Kong IG, Sato A, Kataoka N, Tokuhara D, Kurokawa S, Takahashi Y, Tsukada H, Kozaki S, Akiyoshi K, Kiyono H (2010) Nanogel antigenic protein-delivery system for adjuvant-free intranasal vaccines. Nat Mater 9:572–578
Akiyoshi K, Ueminami A, Kurumada S, Nomura Y (2000) Self-association of cholesteryl-bearing poly(l-lysine) in water and control of its secondary structure by host–guest interaction with cyclodextrin. Macromolecules 33:6752–6756
Akiyoshi K, Kang EC, Kurumada S, Sunamoto J, Principi T, Winnik FM (2000) Controlled association of amphiphilic polymers in water: thermosensitive nanoparticles formed by self-assembly of hydrophobically modified pullulans and poly(N-isopropylacrylamides). Macromolecules 33:3244–3249
Hirakura T, Nomura Y, Aoyama Y, Akiyoshi K (2004) Photoresponsive nanogels formed by the self-assembly of spiropyrane-bearing pullulan that act as artificial molecular chaperones. Biomacromolecules 5:1804–1809
Akiyoshi K, Sasaki Y, Kuroda K, Sumanoto J (1998) Controlled association of hydrophobized polysaccharide by cyclodextrin. Chem Lett 27:93–94
Sendtner M, Carroll P, Holtmann B, Hughes RA, Thoenen H (1994) Ciliary neurotrophic factor. J Neurobiol 25:1436–1453
Chen BL, Arakawa T (1996) Stabilization of recombinant human keratinocyte growth factor by osmolytes and salts. J Pharm Sci 85:419–422
Chen BL, Arakawa T, Hsu E, Narhi LO, Tressel TJ, Chien SL (1994) Strategies to suppress aggregation of recombinant keratinocyte growth factor during liquid formulation development. J Pharm Sci 83:1657–1661
Sharma L, Sharma A (2001) Influence of cyclodextrin ring substituents on folding-related aggregation of bovine carbonic anhydrase. Eur J Biochem 268:2456–2463
Machida S, Ogawa S, Shi X, Takaha T, Fujii K, Hayashi K (2000) Cycloamylose as an efficient artificial chaperone for protein refolding. FEBS Lett 486:131–135
Nomura Y, Ikeda M, Yamaguchi N, Aoyama Y, Akiyoshi K (2003) Protein refolding assisted by self-assembled nanogels as novel artificial molecular chaperone. FEBS Lett 553:271–276
Asayama W, Sawada S, Taguchi H, Akiyoshi K (2008) Comparison of refolding activities between nanogel artificial chaperone and GroEL systems. Int J Biol Macromol 42:241–246
Sawada S, Nomura Y, Aoyama Y, Akiyoshi K (2006) Heat shock protein-like activity of nanogel artificial chaperone for citrate synthase. J Bioact Compat Polym 21:487–501
Rozema D, Gellman SH (1995) Artificial chaperones: protein refolding via sequential use of detergent and cyclodextrin. J Am Chem Soc 117:2373–2374
Cleland JL, Hedgepeth C, Wang DIC (1992) Polyethylene glycol enhanced refolding of bovine carbonic anhydrase B. Reaction stoichiometry refolding model. J Biol Chem 267:13327–13334
Shimizu H, Fujimoto K, Kawaguchi H (2000) Renaturation of reduced ribonuclease A with a microsphere-induced refolding system. Biotechnol Prog 16:248–253
Dong XY, Wang Y, Shi JH, Sun Y (2002) Size exclusion chromatography with an artificial chaperone system enhanced lysozyme renaturation. Enzyme Microb Technol 30:792–797
Morimoto N, Endo T, Ohtomi M, Iwasaki Y, Akiyoshi K (2005) Hybrid nanogels with physical and chemical cross-linking structures as nanocarriers. Macromol Biosci 5:710–716
Bloxham DP, Ericsson LH, Titani K, Walsh KA, Neurath H (1980) Limited proteolysis of pig heart citrate synthase by subtilisin, chymotrypsin, and trypsin. Biochemistry 19:3979–3985
Avrameas S, Guilbert B (1972) Enzyme-immunoassay for the measurement of antigens using peroxidase conjugates. Biochimie 54:837–842
Smith AT, Santama N, Dacey S, Edwards M, Bray RC, Thorneley RN, Burke JF (1990) Expression of a synthetic gene for horseradish peroxidase C in Escherichia coli and folding and activation of the recombinant enzyme with Ca2+ and heme. J Biol Chem 265:13335–13343
Doyle WA, Smith AT (1996) Expression of lignin peroxidase H8 in Escherichia coli: folding and activation of the recombinant enzyme with Ca2+ and hem. Biochem J 315:15–19
Breslow R, Zhang BL (1996) Cholesterol recognition and binding by cyclodextrin dimers. J Am Chem Soc 118:8495–8496
Harada A, Adachi H, Kawaguchi Y, Kamachi M (1997) Recognition of alkyl groups on a polymer chain by cyclodextrins. Macromolecules 30:5181–5182
Nomura Y, Sasaki Y, Takagi M, Narita T, Aoyama Y, Akiyoshi K (2005) Thermoresponsive controlled association of protein with a dynamic nanogel of hydrophobized polysaccharide and cyclodextrin: heat shock protein-like activity of artificial molecular chaperone. Biomacromolecules 6:447–452
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research from the Japanese government (nos. 20240047 and 18GS0421).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sawada, Si., Sasaki, Y., Nomura, Y. et al. Cyclodextrin-responsive nanogel as an artificial chaperone for horseradish peroxidase. Colloid Polym Sci 289, 685–691 (2011). https://doi.org/10.1007/s00396-010-2361-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-010-2361-0