Abstract
The effect of some amphipilic diblock-copolymers and comb-polymers on a balanced Winsor III microemulsion system is investigated with the quaternary system n-octyl-β-d-glucoside/1-octanol/n-octane/D2O as basis system. The diblock-copolymers are polyethyleneoxide-co-polydodecenoxide (PEO x PEDODO y ) and polyethyleneoxide-co-polybutyleneoxide (PEO x PEBU y ), constituted of a straight chain hydrophilic part and a bulky hydrophobic part. Addition of the diblock-copolymer leads to an enhancement of the swelling of the middle phase by uptake of water and oil; a maximum boosting factor of 6 was obtained for PEO111PEDODO25. Nuclear magnetic resonance diffusometry yields the self-diffusion coefficients of all the components in the system. The diffusion experiments provide information on how the microstructure of the bicontinuous microemulsion changes upon addition of the polymers. The reduced self-diffusion coefficients of water and oil are sensitive to the type of polymer that is incorporated in the film. For the diblock-copolymers, as mainly used here, the reduced self-diffusion coefficient of oil and water will respond to how the polymer bends the film. When the film bends away from water, the reduced self-diffusion of the water will increase, whereas the oil diffusion will decrease due to the film acting as a barrier, hindering free diffusion. The self-diffusion coefficient of the polymer and surfactant are similar in magnitude and both decrease slightly with increasing polymer concentration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microemulsions are thermodynamically stable, homogeneous liquids and consist of oil, water and surfactant, and often a fourth component such as salt (for ionic surfactants) or a cosurfactant (such as an alcohol in the case of nonionic surfactants). Microemulsions can be characterized, on the basis of their microstructure, into three main types: oil-in-water, water-in oil, and bicontinuous.
The study of microemulsions mixed with polymers is of increasing interest from a scientific and industrial point of view. The addition of small amounts of an amphiphilic diblock-copolymer has been shown to increase the solubilization capacity of water and oil [1–3], sometimes extensively such that, as a consequence, less surfactant is needed to obtain a one-phase microemulsion with given amounts of water and oil, which makes the microemulsion more efficient.
From a scientific standpoint, it is interesting to investigate these synergetic effects, trying to fully understand the mechanism underlying the swelling and the adsorbing of the polymer to the monolayer interface. Such studies, both on classical nonionics and nonionic alkylglucosides (APGs), have been performed by Jakobs et al. [1, 3]. However, in this paper, we extend these studies by investigating commercially available diblock-copolymers with somewhat less hydrophobic blocks and comparing them to the polyethyleneoxide–polyetylpropylene (PEO x PEP y ) made by the group in Jülich (Allgaier et al. [4]). The combination of nonionic glucoside surfactants and diblock-copolymers may constitute useful formulations in the future because of the already mentioned need for less surfactant and the fact that the APGs have good environmental properties such as good biodegrability [5], low toxicity [6–8], and appearing to be less temperature sensitive than the common C i E j surfactants [9].
The objective of the present paper is twofold. Firstly, we study the changes inside the Winsor III region with a focus on swelling as a function of polymer concentration. Secondly, our objective is to study the self-diffusion of a polymer in a monolayer film and to investigate to what extent it changes the behavior of the other components in the film. Kabalnov et al. [10] have studied the self-diffusion of C12E5, oil, and water in a bicontinuous microemulsion system consisting of C12E5/C10H22/H2O/HM-EHEC and found that the curvature changed and curved towards the oil, causing the self-diffusion of the water to increase modestly, while the self-diffusion of the oil decreased modestly. This was reflected in the changes in the water-to-oil ratio.
Experimental section
Materials
Two different surfactants were studied in this work: n-octyl-β-d-glucoside (C8G1) with a purity of 99.5% from Anatrace (Maumee, OH, USA) and n-octyl-triethyleneoxide (C8E3) with a purity of 99% from Nikko Chemicals (Tokyo, Japan). Oil n-octane was used with a purity of 99% from Chemtronica (Stockholm, Sweden), while the cosurfactant 1-octanol (C8E0) was purchased from BDH (Poole, UK) with a purity of 98% and the deuterated 1-octanol (CD3(CD2)7OH) with a purity of 97% was from Cambridge isotope laboratories (MA, USA). The heavy water and deuterated toluene (CD5CD3) used was from Dr. Glaser AG (Basel Switzerland, isotopic purity of 99.5%) and the water was obtained from a Milli-Q Gradient A10 machine. All the substances were used without further purification.
The different polymers used in this study (see Fig. 1), are manufactured by Akzo Nobel AB, Stenungsund, Sweden. The diblock-copolymers consist of a hydrophilic polyethyleneoxide (PEO) part and a hydrophobic poly(1-dodeceneoxide) or poly(butylenoxide) part (see Table 2). The comb-polymer (Ketjenlube) is a polymer with grafted PEO groups and C12/C14 chains onto the backbone (cf. Fig. 1c). The polymers were used as received without further purification. Nuclear magnetic resonance (NMR) proton self-diffusion measurements were also performed with the polymers in deuterated toluene to measure the distribution in self-diffusion coefficients (see Table 2).
Sample preparation
Samples were prepared in 5-mm NMR tubes by adding the desired amount of polymer into a specific composition of a sample in the Winsor III region with a composition of \(\gamma = {\widetilde{\gamma }} \mathord{\left/ {\vphantom {{\widetilde{\gamma }} 2}} \right. \kern-\nulldelimiterspace} 2\), where γ is the overall mass fraction of surfactant (C), cosurfactant (D) and polymer (P) (see Eq. 1, A stands for water while B stands for oil) and \(\widetilde{\gamma }\) corresponds to the fish tail point of the original system without polymer. The minimum amount surfactant and cosurfactant needed to solubilize equal volumes of oil and water (without addition of polymer) is \(\widetilde{\gamma } = 0.1609\)for the C8G1/C8E0/n-octane/D2O system [11].
The volume fraction of oil on the basis of sample oil and water amount was 0.5 (corresponding to 0.3887 wt% for the oil). The samples were flame-sealed, mixed by centrifugation, and subsequently left for equilibration by slow tilting end-over-end at room temperature for at least 12 h before measurements were performed. The occurrences of anisotropic phases were investigated in transmitted light between crossed polarizers to observe birefringence. The relative volumes of the coexisting phases were measured using a ruler (the error in the measurements of the heights is less than 1 mm, and the typical height measured is 90 mm).
The mass composition of the polymer in the film (middle phase and microemulsion phase), δ film, is defined by the ratio between polymer and total amphiphile amount in the film (i.e., cosurfactant, surfactant, and polymer in the film) [1]. The alcohol is somewhat soluble in the oil phase and correction is made for this [11], with the assumption that the polymer does not affect the alcohol solubility in the oil phase. All surfactant is assumed to go to the middle phase. The solubility of the polymer in water and oil is to be found in Table 1; no correction is made for this.
NMR measurements
To investigate how the polymer influences the monolayer film in the microemulsion system, self-diffusion NMR measurements were performed. A Bruker DMX 200 NMR spectrometer, equipped with a field gradient probe unit, was used to measure the self-diffusion coefficients of the components by following the 1H NMR signal at 298±0.5 K.
The stimulated echo–pulse sequence was used following the recommendation in [12, 13]. The self-diffusion coefficients D (or D 1 and D 2 for the case of two overlapping peaks) were obtained by nonlinear least-square fitting of Eq. 3 or 4 to the obtained NMR data.
In Eqs. 3 and 4, I denotes the observed intensities, I 0 is the intensity in absence of gradient pulses, γ is the magnetogyric ratio, G is the gradient strength, δ is the duration time of the gradient pulse, and Δ is the time between the leading edges of the field gradient pulses. When two peaks overlap with different self-diffusion coefficients, for example, for oil and surfactant, the NMR data give biexponential decay. In Eq. 4, D 1 and D 2 are the diffusion coefficients of the two species and P 1 is the fraction of component 1.
A problem in these five-component microemulsion systems is the fact that protons from different constituents have the same chemical shift, so the peaks overlap. Another difficulty arises as the polymer is slightly polydisperse, giving a distribution of self-diffusion coefficients. The peak from water (HDO) and the signal from ethylene oxide (EO) were fully resolved (a spectrum is reproduced in Fig. 2). Upon increasing the values of G 2, the NMR signal intensity from the water peak was single exponential (cf. Eq. 3) while the exponential fitting of the NMR data from the EO peak was less good due to the fact that the polymer is polydisperse and gives instead a distribution of self-diffusion coefficients. The NMR signal from the EO peak will be approximated as single exponential; the error of this approximation is small as can be seen from a typical decay shown in Fig. 3.
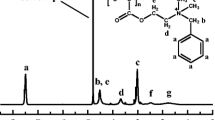
Proton NMR spectrum from a sample of C8G1/C8E0-d17/n-octane/D2O:H2O (95:5)/PEO43PEDODO41. From left to right, the peaks are as follows: HDO peak at 4.7 ppm, EO peak (polymer) at 3.6 ppm, surfactant peak at 3.3 ppm (see inserted spectrum), and, finally, the multicomponent peak at 1.2 ppm that contains contribution from four species
To gain information about the self-diffusion coefficients for the surfactant, one of the peaks of the glucose (at 3.3 ppm) was examined, and a biexponential fitting was performed. The reason for this is that there is overlap from 1-octanol. The oil peak (at 1.2 ppm) contains contributions from four different components. As oil diffuses approximately one order of magnitude faster than the surfactant, 1-octanol and polymer, a biexponential fitting using Eq. 4 was performed to the NMR data, and the fast self-diffusion coefficient was identified as that for oil.
For the NMR measurements of the microemulsion, samples were prepared in 5-mm NMR tubes. The middle microemulsion phase was transferred to a 5-mm NMR tube. For the NMR measurements of the microemulsion with polymer added, 95:5 D2O to H2O ratio instead of pure D2O was used to be able to follow the water signal. To facilitate the interpretation of the self-diffusion NMR data, deuterated 1-octanol was used in some cases. Corrections in compositions were made when deuterated 1-octanol was used instead of normal 1-octanol so that the molar composition was kept constant.
To estimate the polydispersity of the polymers, self-diffusion was investigated in deuterated toluene at a concentration of 1 wt% (where the polymer is expected to be molecularly dispersed). The NMR signal amplitudes were fitted with a nonlinear least-square fitting procedure as recommended in [14] and the obtained result is a log-normal distribution of self-diffusion coefficients. The results of the NMR measurements gave the mean self-diffusion coefficients for both the hydrophilic and their hydrophobic part and its distribution, σ (see Table 2). Applying scaling relations for the self-diffusion coefficients and molecular weight as described in [14] yields a value of the polydispersity M w/M n of 1.4–1.6. The self-diffusion coefficients from the hydrophilic part and from the hydrophobic part of the polymer differ slightly, which could indicate the existence of some unreacted species of the blocks.
General background information
Short review of the phase behavior of microemulsions
The weak temperature dependence of the alkylglycoside surfactant [9] makes it difficult to tune the spontaneous curvature of the surfactant film in the ternary water/n-alkane/C m G n system with temperature to achieve, for example, a bicontinuous microemulsion. The reason for this effect is presumably that the headgroup hydrations of the sugar surfactants respond only moderately to temperature changes.
The key quantities in describing the phase behavior are instead the composition of the internal film [11, 15] after addition of a cosurfactant. The ternary system water/n-alkane/C m G n was tuned by the addition of 1-octanol and, thus, water-in-oil and oil-in-water microemulsion phases could be obtained. The fourth component serves two purposes: (a) The hydrophobic alcohol acts as a cosolvent, making the oil more polar. (b) It increases the effective hydrophobicity of the surfactant film, acting as a cosurfactant.
A useful way to characterize a quaternary system, such as water/n-alkane/C m G n /cosurfactant at equal volumes of water and oil is to do a vertical cut through the phase prism. In this type of representation, the coexistence curves display a fish shape; hence, the term “fish diagram” is often used. At low amount of cosurfactant, a microemulsion of oil-in-water type coexist with an excess oil phase (often denoted \(\underline{2}\)). At higher amount of cosurfactant, a water-in-oil microemulsion exists with an excess water phase (often denoted \(\overline{2}\)). At intermediate concentrations of cosurfactant, a three-phase coexistence with a water excess phase, a microemulsion phase (often of a bicontinuous type), and an oil excess phase is present.
Polymer decorated films
The behavior of microemulsions mixed with polymers depends on their interaction with the monolayer, separating the oil and the water. In general, one can have three different situations: adsorbing polymers, polymers that adsorb but do not mix (see below), and non-adsorbing polymers [16, 17]. The first category of the polymers can mix randomly with the surfactants in the monolayer films. Examples of such polymers are HM-EHEC, diblock-copolymer [such as poly(ethylenoxide)–poly(butylmethacrylate), poly(ethylenoxide)–poly(styrene), poly(ethylenoxide)–poly(ethylene-co-propylene) [2], and the silicone type of polymers (Si x C3EO y [3]). The second class of adsorbing polymers adsorbs to the film but does not mix fully with the surfactant, forming polymer-rich patches on the film [18]. The third class of polymers are those which do not adsorb, e.g., homo-polymers such as EHEC [10], dextran, and poly(isobutylene) [19].
With respect to the size of the polymer, as quantified by the radius of gyration, non-adsorbing homo-polymers (like EHEC and dextran) must have a radius of gyration that is smaller than the pore size of the bicontinuous microemulsion for the polymer to be soluble in the middle phase [10, 16].
Polymers at the interface in a bicontinuous microemulsion can behave very differently depending on their chemical nature and structure, which is reflected in how hydrophilic and how hydrophobic the blocks are. The blocks are reported to form “mushrooms” at lower polymer concentrations while at higher concentrations, though the mean distance between the anchored polymers is smaller than the radius of the polymer coil, the polymers overlap and form a so-called brush regime [20]. In the brush regime, the polymers are stretched out towards the continuous medium due to the repulsive, lateral interaction between the neighboring polymer chains.
When the polymer is highly surface active, it will decorate the surface in a flat manner, often called “pancake” [21]. A possible example of this is a comb-polymer with repeating hydrophilic and hydrophobic side chains (see Fig. 1c) as will be discussed later.
Finally, some polymers anchored to surfaces may form trains and loops [22], depending on the solvent quality.
Flexible surface model
The flexible surface model is a valuable theoretical tool in analyzing and understanding the curvature of self-assembly structures of surfactant systems. It is based on a description of the monolayer as a geometrical surface. When the monolayer deviates from some intrinsic specific value for the given monolayer, the curvature free energy (G c) changes [23, 24] according to Eq. 5:
Here \(H = \frac{1} {2}{\left( {\frac{1} {{R_{1} }} + \frac{1} {{R_{2} }}} \right)}\) and \(K = \frac{1} {{R_{1} R_{2} }}\) are the mean and Gaussian curvatures, respectively. R 1 and R 2 are the two principal radii of curvature, H 0 is the spontaneous curvature, d s is the surface area of the monolayer patch, κ is the bending modulus, and \(\overline{\kappa }\)is the saddle splay modulus.
Theoretical studies of the addition of adsorbing polymer to fluid membranes have been performed [20, 21]. The main conclusions of this work will be described below. By anchoring polymers to a monolayer film, the elastic properties of the film will respond and adapt to the interactions with the polymer. If we focus on a polymer with the copolymer end attached to the film, the film curvature will respond by either bending towards or away from the polymer depending on whether there is an attractive or repulsive interaction between the film and the polymer. For the repulsive case, the surface bends away and the polymer gains configurational entropy (in the mushroom regime). This gain in entropy has been calculated by Hiergeist and Lipowsky [20] by comparing polymers anchored to a curved surface relative to the polymers anchored to a flat surface. In the mushroom and also in the brush regime, the polymers increase the bending rigidity (κ), whereas the saddle splay modulus (\(\overline{\kappa }\)) is reported to decrease [20].
We turn our attention next to the parameters in the flexible surface model which will be modified when a polymer is anchored to the film. Gompper et al. [25] developed a new approach to determine the bending rigidity and saddle splay modulus of the amphiphilic film in microemulsions and sponge phases by using a neutron scattering experiment with sophisticated contrast matching. The effect of polymer decoration on membrane elasticity can be written as a sum of terms, a contribution related to the polymer free system plus a second term that takes explicitly the effect of polymer interaction on the system into account as described by [25–28]:
where κ and \(\overline{\kappa }\) are the bending modulus of the pure surfactant membrane and the effective parameters (after polymer addition) are κ eff, the effective bending rigidity, \(\overline{\kappa } _{{eff}}\), the effective saddle splay modulus, and H 0,eff, the effective spontaneous curvature. σ is the number density of the diblock-copolymer within the membrane and R a/R b are the end-to-end distances of the hydrophilic/hydrophobic block. In the brush regime, the presence of the polymer increases the elastic curvature even more than in the mushroom regime and the dependence on σ is expected to be approximately cubic [20].
Solubility of the polymers in water and oil
The polymers discussed in this paper are amphiphilic in nature, i.e., they are surface active (enrich at interfaces) and they have the ability to form micelles in an aqueous water phase and reverse micelles in an oil phase. As a consequence, they have also the ability to interact with monolayer surfactant films at the oil/water interface. To obtain maximum solubilization enhancement of water and oil (i.e., boosting effects), it is preferable that the solubility of the polymers in both oil and water is low (depending on the molecular architecture, diblock-copolymers have a preference to be more soluble in either the oil or water continuous phases). The value of the amount of polymer in the film, δ film, is overestimated because in the calculation of δ film we have assumed that all polymers sit in the microemulsion phase and no polymers are dissolved in the water phase or the oil phase.
The oil phase (or water phase) with dissolved polymers will expand at the expense of the middle phase (microemulsion phase) but, on the other hand, the middle phase with polymers in the film will have a modified curvature according to Eqs. 6, 7, and 8 and, therefore, swell. These two effects operate against each other; so, if the polymer dissolves in the oil or water phase, the boosting effect will be somewhat quenched. The solubilization of polymers (for instance, homopolymers as a byproduct from the synthesis of, e.g., diblock-coplymers) in the water or oil phase decreases the efficiency strongly. This effect is called anti-boosting effect and has been discussed by Byelov et al. [29].
The molecular weights of the different blocks are important in the discussion of the swelling of the microemulsion. If, for example, the hydrophobic block is much larger than the hydrophilic block, the solubility of the diblock-copolymer in oil is likely to increase in comparison to a diblock-copolymer that is in a balanced state.
The film area available for the polymer is also important. Addition of, for example, a HM-end grafted polymer can saturate the film. Kabalnov et al. [10] defined a polymer partition coefficient (r) for HM-EHEC and found a maximum in the partition coefficient at a certain value of polymer concentration, at which value of r the polymer efficiency in terms of swelling of the microemulsion was at a maximum.
Results
In this work, we consider two different types of diblock-copolymer and one comb-polymer. The polymer solubilities in water and oil are given in Table 1. For the polymers used in this work, we do not need to consider the solubility in water of the polymer because the water solubility is very low but, on the other hand, the oil solubilities are somewhat higher.
The polymers could be characterized with respect to their behavior in the film. Figure 4 is a schematic illustration of how the different polymers may interact with the film. In case 1, the polymer stretches out in both the oil and the water domains, while in case 2, the hydrophilic part stretches out in the water phase while the hydrophobic part adsorbs to the film (of course, the reverse case is also possible). Typical examples of case 1 polymers are diblock-copolymers that have one hydrophilic block and one hydrophobic block. A case 2 polymer could also be a diblock-copolymer (as it is for some of our polymers, as will be discussed later). In case 3, finally, the polymer is lying flat onto the film (“pancake state”). An example of case 3 would be a comb-polymer with its hydrophilic and hydrophobic tails grafted onto the backbone of the polymer sticking out into the water/oil domains, respectively.
Schematic illustration of the microemulsion with the polymer anchored to the film in three different manners. In case 1, the diblock-copolymers are in the mushrooms regime with the hydrophilic/hydrophobic part sticking out into the water and oil domains, in case 2, the hydrophilic part of the diblock-copolymer is sticking out into the water domain while the hydrophobic part is lying flat along the film, and, finally, in case 3, the polymers are lying flat on the monolayer film with the small hydrophilic/hydrophobic stickers possibly sticking out into the water/oil domains, respective
Swelling behavior
Investigation of polymer addition to the C8G1/C8E0/n-octane/H2O system
The system used here to investigate the swelling behavior is the quaternary system n-octyl-β-d-glucoside/1-octanol/n-octane/D2O at equal volume fractions of water and oil [11]. Figure 5 shows the swelling behavior of the different polymers as a function of the mass fraction of the polymer in the film (please note that the C8E3 system contains no cosurfactant). The swelling has been determined visually. The relative volumes of the different phases have been determined by measuring the phase’s size with a ruler.
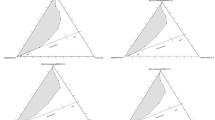
Relative volumes of the different phases. a–e The system C8G1/1-octanol/n-octane/D2O with the mass fraction 4.5/2.5/36/54 is under study. e,f The system C8E3/C8E0/H2O with the mass fraction 7/39/54 is under study. The lower area section is the water excess phase, the middle section is the microemulsion phase, and the top section is the oil excess phase. δ film indicates the polymer concentration in the film. The bottom areas at high δ film in (b) indicate the presence of a lamellar phase
A sample in three-phase region of a fish diagram contains three phases, where the middle phase is a microemulsion, which grows in volume upon addition of surfactant by incorporating more oil and water, although the mesh size of the water and oil domains remain constant in a bicontinuous microemulsion. Addition of a diblock-copolymer, on the other hand, alters the mesh size when the microemulsion responds by swelling water and/or oil. We will discuss below the influence of the different polymers.
The PEOxPEDODOy system
Adding the polymer causes the middle phase to increase in volume. This increase is nearly symmetric (the decrease in volume of oil and water are the same), but a closer inspection (Fig. 5a,b) shows that this is only true at low polymer concentrations, whereas at higher polymer concentrations, either oil or water disappears first, depending on the molecular weights of the blocks. A plausible explanation of the differences of the swelling behavior is that the blocks penetrate to a different extent into the oil or water domains, which reflect how hydrophobic/hydrophilic they are. The decrease in the water and oil volumes should be unequal according to the curvature model laws expressed in Eqs. 6 and 7 when R a≠R b. The spontaneous curvature will deviate from zero curvature, H 0=0, when R a≠R b and curve slightly towards oil (or water) and have either a positive or a negative curvature according to Eq. 8.
A way to define how efficient the polymer is in solubilizing water and oil is to define a boosting factor, f B [1]:
Equation 9 relates how much surfactant plus cosurfactant is replaced \(\widetilde{\gamma } - \gamma {\left( {1 - \delta _{{{\text{film}}}} } \right)}\)by the diblock-copolymer,\(\gamma \delta _{{film}} .\) The values used for the calculation of the boosting factors for the polymers were \(\widetilde{\gamma } = 0.1609\), γ=0.098, δ film=0.3 (Fig. 5a), and γ=0.089, δ film=0.22 which gave the boosting factors of ƒ B=3 and ƒ B=5, respectively (for comparison, see Jakobs et al. [2]). A boosting factor can only be calculated for those polymers that cause the microemulsion to swell by taking up all the oil and water. The difference between the polymers studied here and the one Jacobs et al. [1, 2] used is in the hydrophobic part. It would be interesting to compare the extended length of the hydrophobic part because the ones they used are of a straight-chain type while the ones we used are much more bulky (with a monomer with a C12-chain grafted onto the backbone on the hydrophobic part of the polymer, see Fig. 1a). The end-to-end distance of the hydrophobic part of the straight-chain type is greater than that of the bulky type, everything else is equal. As noted above, the length which the respective part stretches out in water and oil is probably one of the more important factors in achieving an effective boosting factor.From Fig. 5b it is clear that a lamellar phase is formed when δ film exceeds a value of 0.22. Jakobs et al. [1] noticed that, at lower content of the diblock-copolymer PEOPEP (δ film >0.1), the lamellar phase is suppressed. However, at higher contents, this trend reverses and a lamellar phase is formed [1, 30], in agreement with the results obtained here. Kumar et al. [3] reported an enhancement of the solubilization efficiency when they used Si x C3EO y in the system C12E5/n-dodecane/H2O, but the polymer addition here also induced the formation of the lamellar phase (both the one-phase region and the lamellar phase were shifted to the left in the fish phase diagram).
The PEOxPEBUy system
The microemulsion phase (the middle phase) expands here in volume as a function of δ film by incorporating mostly water; the swelling is highly antisymmetric (see Fig. 5c,d). For the PEO x PEDODO y system discussed above, both blocks penetrate into the water and oil phase, respectively, and this leads to a case 1 (see Fig. 4, left picture) situation, while the PEO x PEBU y system is more likely to be a representative of case 2 (see Fig. 4, middle picture). For the PEO x PEDODO y system, the oxygen is screened by ten methylene groups, whereas in the PEO x PEBU y , system it is only screened by three, which makes this polymer less hydrophobic than the previous one. It is likely that the hydrophobic block partly lies flat in the film or has some part anchored to the film (in the form of trains and loops). We note that Endo et al. [27, 28, 31] measured the distribution of the PEPPEO in the film and found that the polymers form uniformly distributed “mushrooms”.
The Ketjenlube system
Addition of Ketjenlube alters the phase behavior weakly (see Fig. 5e). The oil volume is approximately constant, while the water volume decreases slightly upon addition of polymers. The reason for the low efficiency in swelling is that the hydrophilic/hydrophobic stickers are short and that the grafting density of the stickers on the polymer backbone is rather low.
As the water/oil solubility is high for Ketjenlube, a fraction of the polymer is distributed in water and oil phase, respectively, decreasing the efficiency of the polymer.
In summary, it is only the PEO111PEDODO25 and PEO43PEDODO41 systems that give an enhancement of swelling of the microemulsion for both oil and water. The other polymers, PEO43PEBU106, PEO111PEBU67, and Ketjenlube, only give a significant enhancement of water swelling in the microemulsion.
Investigation of polymer addition to the C8E3/n-octane/H2O system
So far, only one microemulsion system (C8G1/C8E0/n-octane/H2O) has been analyzed with respect to addition of the polymers. We now focus our attention on the system C8E3/n-octane/H2O to investigate if the studied polymers will swell a microemulsion, based on a surfactant, which has temperature as a tuning parameter. The temperature was set to 16°C (the temperature when the surfactant film does not have a preference to curve either towards water or oil).
Only the PEO x PEDODO y class will be investigated, as this polymer gave an enhancement in solubilization of water and oil with the alkylglucoside surfactant system. The swelling effects the diblock-copolymers have on the system are reported in Fig. 5f,g. As can be seen from Fig. 5f,g, swelling occurs at the expense of water and oil. It is interesting to compare it with Fig. 5a,b where a complete solubilization of water and oil occurred. For these systems, we report a boosting factor of 4 and 6, respectively [cf. Fig. 5f,g, where we have used ˜γ=0.1901, γ=0.099, δ film=0.31 (PEO43PEDODO41) and γ=0.089 and δ film=0.22 (PEO111PEDODO25)]. For comparison, the boosting factor was calculated to approximately 15, for the system C8E3/n-octane/H2O/PEOPEP [poly(ethylenepropylene)-co-poly(ethyleneoxid), with molecular weight of 10 kg/mol (5+5 kg/mol)] using the information from the paper by Gompper et al. [25]. Another factor is the possible existence of homo-polymers in the starting material, which would cause the boosting to decrease [29].
NMR measurements
The microstructure of the microemulsion has been studied with the NMR diffusometry technique, which allows one to obtain the self-diffusion coefficients of all the components in the microemulsion system [12, 13].
In a bicontinuous microemulsion, the water and oil domains are assumed to have the same properties as the neat liquids, with self-diffusion coefficients approximately the same as for neat liquids, D 0. It is customary to normalize the self-diffusion coefficients D with the bulk value, D 0. When D/D 0 deviates from unity (neglecting solvation effects), this effect is due to obstruction by the impermeable dividing films. In the balanced state, the ratio D/D 0 is approximately 2:3. In a bicontinuous microemulsion, the geometrical obstruction factor depends on the water-to-oil ratio. It is common to perform an expansion around the balanced state, for water and oil diffusion [12, 32–34]:
where φ 0 is the volume fraction of oil and β is the coordination number. The surfactant self-diffusion is at its maximum for the balanced state, and the corresponding expansion is:
where β′ is an expansion coefficient and \(D^{0}_{{\text{C}}}\) is the lateral self-diffusion of the surfactant along the surfactant film. When the reduced self-diffusion coefficients of the water and oil are equal, the microemulsion is in the balanced state. When the bicontinuous microemulsion is diluted with water, the reduced self-diffusion of water increases whereas the reduced self-diffusion of oil decreases. For the surfactant, the reduced self-diffusion decreases (see Eqs. 10, 11, and 12). The interpretation of a difference in reduced self-diffusion coefficients of oil and water in a microemulsion is that the spontaneous curvature deviates from zero (bends towards water or oil) or that the water or oil has specific interactions with surfactants in the film. The situation for the microemulsion will be different when the volume of the film decreases, as will be discussed later.
Oil and water self-diffusion
The self-diffusion of water and oil were normalized with the corresponding quantities obtained from a polymer-free system to make it possible to compare the polymer-free microemulsion with systems with polymer. For the microemulsion system containing PEO43PEDODO41 (see Fig. 6a), both D A and D B increase slightly upon addition of PEO43PEDODO41. The most noteworthy conclusion from Fig. 6a is that the reduced self-diffusion of water and oil tend towards the same reduced diffusion value at δ film=0.26. The interpretation of this increase is that the water and oil mesh size has grown larger and has a maximum at δ film=0.26 (note from Fig. 5a that a value of δ film=0.26 approximately corresponds to a one-phase sample). A second interesting observation is that the reduced self-diffusion coefficient for oil reaches a maximum value at 0.18 which corresponds to the value of δ film in the swelling experiment where the microemulsion has taken up all the oil (cf. Fig. 5a). Thus, the self-diffusion coefficients of water and oil report on the changes of curvature in the film when polymers are added.
Turning our attention to the PEO111PEDODO25 system, we note that the reduced self-diffusion for water increases with polymer concentration, whereas the oil self-diffusion decreases with polymer concentration (see Fig. 6b), indicating that the polymer has the effect of bending the monolayer toward the oil, thus, making the spontaneous curvature increasingly positive. The reduced water self-diffusion coefficient increases linearly and reaches a maximum value at δ film=0.14, which approximately corresponds to the value of δ film found in the swelling experiments where the microemulsion has swallowed the whole water phase (see Fig. 5b). The reason for the increase in the reduced water self-diffusion is that the domain size of the water has increased.
We turn our interest next to the reduced self-diffusion of water and oil in the systems with PEO111PEBU67 and Ketjenlube. Both for the PEO111PEBU67 and Ketjenlube, the reduced water self-diffusion increases (see Fig. 6c,d) while the reduced self-diffusion for oil decreases. For Ketjenlube and PEO111PEBU67, the increase in the reduced water self-diffusion coefficients is explained by the fact that the microemulsion is curved towards the oil (as discussed above).
The reduced water self-diffusion coefficient for the PEO111PEBU67 systems behaves differently in comparison with, for example the PEO111PEDODO25 reduced water self-diffusion coefficient (compare with Fig. 6b). No plateau is observed i.e. no maximum value for the self-diffusion is found when the water is completely taken up by the microemulsion.
One possible explanation is that the hydrophilic part (more effective in making the hydrophilic domain swelling) dominates the behavior of the curvature and bends it effectively towards oil. If more diblock-copolymer is added, the formation of oil droplets in water is likely to occur.
Finally, for Ketjenlube, the grafted PEO groups on the backbone (approximately seven PEO group in each side arm) are somewhat more effective in swelling water than the grafted hydrophobic chains on the backbone (12–14 methylene groups in each arm) is in swelling oil. The effect this would have is to bend the curvature towards oil as indicated by the diffusion coefficient in Fig. 6d.
The surfactant and polymer self-diffusion in the microemulsion film
The general behavior for the surfactant and polymer is that the self-diffusion decreases upon increasing δ film (see Fig. 7a–c). As background information, it is interesting to calculate the polymer–polymer distance, d pp. Knowing the ratio of polymer to surfactant and cosurfactant and the headgroup area C8G1≈40 Å2 and C8E0≈20 Å2, one can calculate the mean distance between two polymers. For δ film=10, we obtain d pp=40 Å; for δ film=0.20, d pp=27 Å; and for δ film=0.25, d pp=24 Å. Even though d pp is rather small, the polymer and surfactant self-diffusion are similar in magnitude, which might indicate that the film properties influence the self-diffusion of the polymers in the film more than the size of the polymer.
Self-diffusion of surfactant and polymers vs δ film. Four different polymers have been used: a PEO43PEDODO41, b PEO111PEDODO25, and c PEO111PEBU67 and Ketjenlube. In (c), the surfactant self-diffusion is not included as this sample did not contain deuterated C8E0. The lines drawn in the graphs are only guides to the eyes
It is interesting to compute the lateral diffusion of the polymer along the film. This can be done by multiplying the observed value with the factor 3/2 to take into account the dimensionality of the diffusion in the microemulsion [32]. One then obtains for PEO111PEDODO25 a self-diffusion coefficient of 3·10−11 m2s−1. This can be compared with the self-diffusion of a PEG (polyethylene glycol) polymer of the same molecular weight in water which is 7·10−11 m2s−1. Thus, the lateral diffusion is more than a factor of 2 slower than PEG, indicating as mentioned above that it is the interactions in the film that determine the self-diffusion of the polymer in the film.
In Fig. 7c, the self-diffusion of PEO111PEBU67 decreases as a function of δ film. The decrease is steeper than observed for PEO43PEDODO41 and PEO111PEDODO25 (see Fig. 7a,b). This is caused by the fact that the addition of PEO111PEBU67 to the microemulsion induces curvature of the surfactant film towards oil, as discussed above in relation to the self-diffusion of oil and water. Note that the hydrophilic chain is longer than the hydrophobic chain in the case of PEO111PEBU67.
The Ketjenlube self-diffusion (in Fig. 7c) also decreases as a function of δ film. The self-diffusion coefficient is slightly lower than the other polymers, which can be explained by its larger molecular weight. The solubility of the polymer in water is high (see Table 1) indicating that a fraction of the polymer is in the water phase and, therefore, the values δ film are overestimated.
Conclusion
We have demonstrated in this study that an enhancement of solubilization of water and oil could be achieved even if the hydrophobic part of the polymer added is rather branched and not extremely hydrophobic. Another important observation is that only some of the polymers swell by taking up equal volumes of water and oil, while others swell by preferably taking up water or oil. If the polymer is situated perpendicular to the film, there will be an enhancement of swelling, but if the polymer lies flat on the surface, there will be very little swelling effect of water and oil, which is in agreement with what Jacobs et al. [1, 26–28, 31] have reported.
With NMR self-diffusion measurements performed in this study, we analyzed what effects the polymers have on the curvature when polymers were added to the microemulsions. The general observation was that, when the blocks in the diblock-copolymer have big differences in molecular weight, the reduced self-diffusion of either water or oil would be faster depending if it were a hydrophilic block or hydrophobic block that is the larger block. The curvature of the film in the microemulsion responds if there is an imbalance in the size of the blocks in the diblock-copolymer and bends towards the side of the film with the smallest block.
References
Jakobs B, Sottmann T, Strey R, Allgaier J, Willner L, Richter D (1999) Langmuir 15:6707
Jakobs B, Sottmann T, Strey R (2000) Tenside Surfactants Deterg 37:357
Kumar A, Uddin MH, Kunieda H, Furukawa H, Harashima A (2001) J Dispers Sci Technol 22:245
Allgaier J, Poppe A, Willner L, Richter D (1997) Macromolecules 30:1582
Hirsinger F, Schick K-P (1995) Tenside Surfactants Deterg 32:193
Steber J, Guhl W, Stelter N, Schröder FR (1995) Tenside Surfactants Deterg 32:515
Kahare J, Tesmann H (1997) Cosmetics and toiletries manufacture worldwide
Hughes FA, Lew BW (1970) J Am Oil Chem Soc 47:162
Kluge K, Stubenrauch C, Sottmann T, Strey R (2001) Tenside Surfactants Deterg 38:30
Kabalnov A, Olsson U, Thuresson K, Wennerström H (1994) Langmuir 10:4509
Sottmann T, Kluge K, Strey R, Reimer J, Söderman O (2002) Langmuir 18:3058
Söderman O, Stilbs P (1994) Prog Nucl Magn Reson Spectrosc 26:445
Stilbs P (1987) Prog Nucl Magn Reson Spectrosc 19:1
Håkansson B, Nydén M, Söderman O (2000) Colloid Polym Sci 278:399
Reimer J, Söderman O, Sottman T, Kluge K, Strey R (2003) Langmuir 19:10692
Kabalnov A, Lindman B, Olsson U, Piculell L, Thuresson K, Wennerström H (1996) Colloid Polym Sci 274:297
Sottmann T (2002) Curr Opin Colloid Interface Sci 7:57
de Gennes PG (1990) J Phys Chem 94:8407
Kabalnov A, Olsson U, Wennerström H (1994) Langmuir 10:2159
Hiergeist C, Lipowsky R (1996) J Phys II 6:1465
Hiergeist C, Indrani VA, Lipowsky R (1996) Europhys Lett 36:491
Israelachvili J (2002) Intermolecular and surface forces, 2nd edn. Academic
Helfrich W (1973) Z Naturforsch 28c:693
Canham PB (1970) J Theor Biol 26:61
Gompper G, Endo H, Mihailescu M, Allgaier J, Monkenbusch M, Richter D, Jakobs B, Sottmann T, Strey R (2001) Europhys Lett 56:683
Gompper G, Richter D, Strey R (2001) J Phys Condens Matter 13:9055
Endo H, Mihailescu M, Monkenbusch M, Allgaier J, Gompper G, Richter D, Jakobs B, Sottmann T, Strey R, Grillo I (2001) J Chem Phys 115:580
Endo H, Allgaier J, Mihailescu M, Monkenbusch M, Gompper G, Richter D, Jakobs B, Sottmann T, Strey R (2002) Appl Phys A 74:392
Byelov D, Frielinghaus H, Holderer O, Allgaier J, Richter D (2004) Langmuir 20:10433
Frank C, Sottman T, Stubenrauch C, Allgaier J, Strey R (2005) Langmuir 21:9058
Endo H, Allgaier J, Gompper G, Jakobs B, Monkenbusch M, Richter D, Sottmann T, Strey R (2000) Phys Rev Lett 85:102
Anderson MD, Wennerström H (1990) J Phys Chem 94:8683
Lindman B, Shinoda K, Olsson U, Anderson D, Karlström G, Wennerström H (1989) Colloids Surf 38:205
Lindman B, Olsson U (1996) Ber Bunsenges Phys Chem 100:344
Acknowledgements
M. N. and O. S. would like to acknowledge the financial support from the competence center for Surfactant Based on Natural Products, SNAP, and Akzo Nobel Stenungsund for polymer synthesis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Nilsson, M., Söderman, O. & Johansson, I. The effect of polymers on the phase behavior of balanced microemulsions: diblock-copolymer and comb-polymers. Colloid Polym Sci 284, 1229–1241 (2006). https://doi.org/10.1007/s00396-006-1469-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-006-1469-8