Abstract
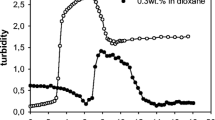
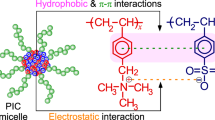
Micelles with azobenzene at the coronas or the cores were prepared by the micellization of ‘nonamphiphilic’ diblock copolymers through hydrogen bond cross-linking. We used 4-(phenylazophenoxymethyl)styrene (AS) as the azobenzene. A poly(vinylphenol)-block-poly(AS-co-styrene) diblock copolymer (PVPh-b-P(AS-co-St)) was prepared by combination of the nitroxide-mediated living radical polymerization and the hydrolysis. The copolymer contained ca. 1 mol% of the azobenzene units in the P(AS-co-St) blocks on the basis of 1H NMR analysis. The PVPh-b-P(AS-co-St) copolymer showed no micellization in 1,4-dioxane, the nonselective solvent. Dynamic light scattering demonstrated that the copolymer formed micelles in the presence of 1,4-butanediamine (BDA) in this solvent. 1H NMR analysis revealed that the azobenzene moieties were located at the coronas of the micelles, because the signals of the aromatic protons originating from the azobenzene had no changes in the shape and the intensity by the micellization. UV analysis supported the presence of the azobenzene at the micellar coronas. The size of the PVPh-b-P(AS-co-St) micelles was independent of the copolymer concentration. On the other hand, the aggregation number of the micelles was dependent not only on the copolymer concentration but also on the kind of the diamine. A poly(AS-co-vinylphenol)-block-polystyrene diblock copolymer (P(AS-co-VPh)-b-PSt) formed the micelles with the azobenzene at the cores of the micelles by BDA. UV analysis demonstrated that the azobenzene at the micellar cores still had the potential to function as photorefractive switching.


















Similar content being viewed by others
References
Imaba Y, Daniels ES, El-Aasser MS (1994) J Coat Technol 66:63
Ha T, Song, H, Lee H, Kim J (2000) Colloids Surf A 162: 289
Lum KK, Campbell BC, Gray ML (2000) Ger Offen DE 10017359 A1 2 Nov, p 10
Bohnel B, Schlosser DL (1991) Eur Pat Appl EP 439941 A1 7 Aug, p 11
Di S, Frank V (1993) Eur Pat Appl EP 534393 A1 31 Mar, p 9
Simpson LA, Robb J, Banford J, Dietz PF, Temperley J (1993) Eur Pat Appl EP 573150 A2 8 Dec, p 14
Yabuta M, Tominaga A, Murata K (1993) Polym Mater Sci Eng 70:168
Schlossman DS (1994) US 5314683 A 24 May, p 12
Bara I, Mellul M (1996) Can Pat Appl CA 2153545 AA 12 Jan, p 18
Pope EJA (1994) J Sol-Gel Sci Technol 2:717
Tuncay M, Calis S, Kas HS, Ercan MT, Peksoy I, Hincal AA (2000) J Microencapsulation 17:145
Ijichi K, Uemura Y, Yoshizawa H, Hatate Y, Haraguchi T, Ide S, Hatanaka C, Yamada K, Kawano Y (1997) Kagaku Kogaku Ronbunshu 23:578
Delair T, Pichot C, Mandrand B (1994) Colloid Polym Sci 272:72
Charleux B, Fanget P, Pichot C (1992) Makromol Chem 193:205
Sugiyama K, Ohga K, Kikukawa K (1994) Macromol Chem Phys 195:1341
Zerfa M, Brooks BW (1997) J Appl Polym Sci 65:127
Wang G, Li M, Chen X (1997) J Appl Polym Sci 65:789
Chen Y, Yang H (1992) J Polym Sci Part A Polym Chem 30:2765
Horak D, Svec F, Frechet JMJ (1995) J Polym Sci Part A Polym Chem 33:2961
Bamnolker H, Margel S (1996) J Polym Sci Part A Polym Chem 34:1857
Taylor MB, Gilbert RD, Stannett VT (1994) J Appl Polym Sci 53:1385
Sun Fuming, Ruckenstein E (1993) J Appl Polym Sci 48:1279
Chern C, Chen Y (1996) Polym J 28:627
Wang, ST, Schork FJ, Poehlein GW, Gooch JW (1996) J Appl Polym Sci 60:2069
Krause S (1964) J Phys Chem 68:1948
Cogan KA, Gast AP (1990) Macromolecules 23:745
Zhu J, Eisenberg A, Lennox RB (1992) Macromolecules 25:6547
Antonietti M, Heinz S, Schmidt M, Rosenauer C (1994) Macromolecules 27:3276
Yoshida E, Wells SL, DeSimone JM (2001) Kobunshironbunshu 58:507
Ismael M, Tondre C (1992) Langmuir 8:1039
Chang Q, Chen (1995) J Chem Eng J 59:303
Yoon KA, Burgess DJ (1997) J Pharm Pharmacol 49:478
Lawrence MJ, Lawrence SM, Barlow DJ (1997) J Pharm Pharmacol 49:594
Aoyama Y, Kanamori T, Nakai T, Sasaki T, Horiuchi S, Sando S, Niidome T (2003) J Am Chem Soc 125:3455
Monaham SD, Wolff JA, Slattum PM, Hagstrom JE, Budker VG (2003) US Pat Appl Publ US 2003027339 A1 6 FEB, p 21
Kwetkat K, Koch H, Ruback W (1997) Ger Offen DE 19524127 A1 9 Jan, p 4
Suzuki Y, Horie M, Okamoto Y, Kurose Y, Maeda S (1998) Jpn J Appl Phys 37: 2084
Suzuki Y, Okamoto Y, Kurose Y, Maeda S (1999) Jpn J Appl Phys 38:1669
Kang H, Lee B, Yoon J, Yoon M (2000) J Colloid Interface Sci 231:255
Orihara Y, Matsumura A, Saito Y, Ogawa N, Saji T, Yamaguchi A, Sakai H, Abe M (2001) Langmuir 17:6072
Buwalda RT, Stuart MCA, Engberts JBFN (2002) Langmuir 18:6507
Nieuwkerk AC, Van K, Ellen JM, Koudijs A, Marcelis ATM, Sudhoelter EJR (1999) Eur J Org Chem 305
Yoshida E, Kunugi S (2002) Macromolecules 35:6665
Yoshida E (2003) Polym J 35:484
Yoshida E (2003) Polym J 35:965
Miyazawa T, Endo T, Shiihashi S, Okawara M (1985) J Org Chem 50:1332
Yoshida E, Kunugi S (2002) J Polym Sci Part A Polym Chem 40:3063
Morrison D, Grabowski EF, Herb CA (1985) Langmuir 1:496
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yoshida, E., Ohta, M. Preparation of micelles with azobenzene at their coronas or cores from ‘nonamphiphilic’ diblock copolymers. Colloid Polym Sci 283, 521–531 (2005). https://doi.org/10.1007/s00396-004-1179-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-004-1179-z