Abstract
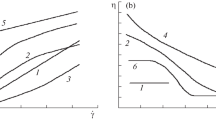
The effect of solely intermolecular interactions due to hydrophobic alkyl substituents on the flow behaviour of hmHEC solutions was determined via comparison of the structure–property relationships of hmHECs and HECs based on the overlap parameter c[η]. For this purpose the η0–[η]–c relationship for HEC was determined to be η0=8.91·10–4+8.91·10–4·c[η]+1.07·10–3(c[η])2+1.83·10–7(c[η])5.56. In addition the structure–property relationship for the longest relaxation time via the λ–[η]–c relationship λ·c1+1/a=2.65·10–8(c[η])2+4.25·10–8(c[η])3+5.44·10–12(c[η])5.27 has been determined. Although the hmHECs had a higher zero shear viscosity than HECs of comparable overlap parameters at a range of 1<c[η]<13, the flow curves could be described via the same λ–[η]–c relationship in that range, indicating a timescale of the intermolecular interactions below the longest relaxation time.
The behaviour of the supramolecular structures in solution with an applied shear field was characterized by rheo-optical analysis of the shear thickening behaviour which occurs with addition of surfactant. Contrary to expectations, a slope >1 of the flow birefringence Δn′ as a function of shear rate could be observed in double logarithmic plotting. The degree of orientation of the flow birefringence φ primarily decreases with increasing shear rate, but increases later on at a characteristic shear rate. These two exceptional phenomena can be explained by a pronounced anisotropy of the polymer coils caused by the dilatant flow.
This assumption is backed up by the occurrence of a maximum in the dichroism curves which is caused by a finite stability of the aggregated structures in solution. On a molecular basis, these observations agree with the theoretically predicted (Witten and Cohen) transition from intra- to intermolecular polymer micelles. The detected aggregates correspond with the polymer chains that are aligned in one micelle.









Similar content being viewed by others
Abbreviations
- a:
-
Exponent of the Mark–Houwink relationship
- c[η]*:
-
Critical concentration (determined by intrinsic viscosity)
- cLS*:
-
Critical concentration (determined by light scattering)
- HASE:
-
Hydrophobically modified alkali-swellable emulsions
- HEUR:
-
Hydrophobically modified ethoxylated urethanes
- hmHEC:
-
Hydrophobically modified hydroxyethylcellulose
- HEC:
-
Hydroxyethylcellulose
- HPMC:
-
Hydroxypropylmethylcellulose
- M:
-
Molecular mass
- MS:
-
Molar degree of substitution
- n:
-
Slope of the flow curve
- SEC:
-
Size exclusion chromatography
- RG :
-
Radius of gyration
- η:
-
Viscosity
- η0 :
-
Zero-shear viscosity
- ηsp :
-
Specific viscosity
- λ:
-
Longest relaxation time
- Δn′:
-
Birefringence
- Δn′i :
-
Intrinsic birefringence
- Δn′f :
-
Form birefringence
- Δn″:
-
Dichroism
- φ:
-
Orientation of the birefringence
- γ̇:
-
Shear rate
References
Graessley WW (1965) J Chem Phys 43:2696
Clasen C, Kulicke WM (2001) Prog Polym Sci 26:1839–1919
Chan AN, Clayton AB, Modi JJ (1998) US Patent 5,804,166
Bolich RE Jr, Norton MJ, Russel GD (1992) US Patent 5,104,646
Majewicz TG, Young TS (1991) US Patent 4,994,112
Bock J, Kowalik RM, Siano DB, Turner SR (1989) US Patent H577
Landoll LM (1985) US Patent 4,529,523
Sau A, Landoll LM, Odell JA, Keller A, Muller AJ (1989) Adv Chem Ser 223:343–364
Goodwin JW, Hughes RW, Lam CK, Miles JA, Warren BC (1989) Adv Chem Ser 223:365–378
Maestro A, Gonzalez C, Gutierrez JM (2002) J Rheol 46:127–143
Nyström B, Thuresson K, Lindman B (1995) Langmuir 11:1994–2002
Panmai S, Prudhomme RK, Pfeiffer DG (1999) Colloids Surf 147:3–15
Piculell L, Nilsson S, Sjösröm J, Thuresson K (1998) Abstr Pap Am Chem Soc 216:025-Cell Part 1
Sivadasan K, Somasundaran P (1990) Colloids Surf 49:229–239
Kaczmarski JP, Tarng MR, Ma Z, Glass E (1999) Colloids Surf 147:39–53
Picullel L, Guillement F, Thuresson K, Shubin V, Ericsson O (1996) Adv Colloid Interface Sci 63:1–21
Kjöniksen AL, Nilsson S, Thuresson K, Lindman B, Nyström B (2000) Macromolecules 33:877–886
Vorobyova O, Winnik MA (2001) Langmuir 17(5):1357–1366
Witten TA Jr, Cohen MH (1985) Macromolecules 18:1915–1918
Ahn KH, Osaki K (1994) J Non-Newtonian Fluid Mech 55:215
Vrahopoulou EP, McHugh AJ (1987) J Rheol 31(5): 371–384
Johnson SJ, Frattini L, Fuller GG (1985) J Colloid Interface Sci 104:440–455
Reinhardt UT, Meyer de Groot EL, Fuller GG, Kulicke WM (1995) Macromol Chem Phys 196:63–74
Baar A, Kulicke WM, Szablikowski K, Kiesewetter R (1994) Macromol Chem 195:1483
Kulicke, WM (1986) Fließverhalten von stoffen und stoffgemischen. Hüthig und Wepf, Heidelberg
Kulicke WM, Kull AH, Kull W, Thielking H, Engelhardt J, Pannek JB (1996) Polymer 37(3):2723–2731
Bueche F (1952) J Chem Phys 20:1959
Bueche F (1956) J Chem Phys 25:599
Simha R, Zakin L (1962) J Colloid Sci 17:270–287
Kulicke WM, Klare J (1980) Angew Makromol Chem 84:67
Bartsch S (1998) Charakterisierung kolloidaler systeme mittels größenausschlußchromatographie, querflußfraktionierung und lichtstreuung. Shaker, Aachen
Kulicke WM (1979) Macromol Chem 180:543
Rouse PE (1953) J Chem Phys 21:1272
Ferry JD (1978) Pure Appl Chem 50:299–308
Ferry JD, Landel RF, Williams ML (1955) J Appl Phys 26:359–362
Ferry JD, Landel RF, Williams ML (1955) J Appl Phys 26:359–362
Kulicke WM, Kniewske R, Müller RJ, Prescher M, Kehler H (1986) Ang Makromol Chem 142:29
Clasen C, Kulicke WM (2001) Rheol Acta 40:74–85
Tam KC, Jenkins RD, Winnik MA, Basset DR (1998) Macromolecules 31:4149–4159
Le Mein JF, Tassin JF, Corpart JM (1999) J Rheol 43:1423–1436
Clasen C, Kulicke WM (2002)J Rheol (in press)
Fuller GG (1995) Optical rheometry of complex fluids. Oxford University Press, New York
Witten TA Jr, Cohen MH (1985) Macromolecules 18:1915–1918
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Laschet, M., Plog, J.P., Clasen, C. et al. Examination of the flow behaviour of HEC and hmHEC solutions using structure–property relationships and rheo-optical methods. Colloid Polym Sci 282, 373–380 (2004). https://doi.org/10.1007/s00396-003-0949-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-003-0949-3