Abstract.
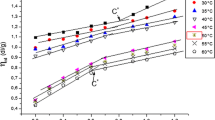
The interactions between poly(vinylpyrrolidone) (PVP) and the reversed micelles composed of water, AOT, and n-heptane are investigated with the aid of phase diagram, measurements of conductivity and viscosity, Fourier transform infrared (FTIR) spectrum, and dynamic light scattering (DLS). The phase diagrams of water/AOT/heptane in the presence of and absence of PVP are given. The conductivity of the water/AOT/heptane reversed micelle without PVP initially increases and then decreases with the increase of water content, ω0 (the molar ratio of water to AOT), while the plots of conductivity (κ) versus ω0 of the reversed micelle in the presence of PVP depend on the PVP concentrations. The plot of κ versus ω0 with 2.0%wt PVP is similar to that without PVP. Only the ω0,max (the water content that the maximum conductivity corresponds to) is larger than that without PVP. Nevertheless, the conductivity of the reversed micelle containing more than 4%wt PVP always rises with the increase of the water content in the measured range. The DLS results indicate that the hydrodynamic radius (Rh) in the presence and absence of PVP rises with the increase of ω0. The plots with PVP and without PVP have almost the same value when ω0<17; and after that, it quickly increases with the increase of ω0. It is interesting to find that there is almost no effect of the PVP concentration on the viscosity and Rh of the reversed micelle at ω0=15. The FTIR results suggest that the contents of SO3 --bound water and Na+-bound water both decrease with PVP added, while the content of the bulky-like water increases. However, the trapped water in the hydrophobic chain of the surfactant is nearly unaffected by PVP. It is also found from the FTIR that the carbonyl group stretching vibration of AOT is fitted into two sub-peaks, which center at 1740 and 1729 cm-1, corresponding to the trans and cis conformations of AOT, respectively.











Similar content being viewed by others
References
Temsamani MB, Maeck M, Hassani IEI, Hurwitz HD (1998) J Phys Chem B 102:3335
Martin CA, Magid LJ (1981) J Phys Chem 85:3938
Casado J, Izquierdo C, Fuentes S, Moyá ML (1994) J Chem 71:446
Schulman JH, Stoeckenius W, Prince LM, (1959) J Phys Chem 63:1677
De TK, Maitra A, (1995) Adv Colloid Interface Sci 59:95
Pileni MP (1993) J Phys Chem 97:6961
Pileni MP (1993) Adv Colloid Interface Sci 46:139
Cui ZG, Yin FS (1999) Emulsifying technology and application. Light Industry Press of China, p 90
Haandrikman G, Daan GJR, Kerkhof FJM, Van Os NM, Rupert LAM (1992) J Phys Chem 96:9061
D'Angelo M, Onori G, Santucci A (1993) J Phys Chem 98:3189
Onori G, Santucci A (1993) J Phys Chem 97:5430
Li Q, Weng SF, Wu JG, Zhou NF (1998) J Phys Chem 102:3168
Li Q, Li T, Wu JG, Zhou NF (2000) J Colloid Interface Sci 229:298
Zhou NF, Li Q, Wu JG, Chen J, Weng SF, Xu GX (2001) Langmuir 17:4505
MacDonald H, Bedwell B, Gulari E, (1996) Langmuir 2:704
Jain TK, Varshney M, Maitra A, (1989) J Phys Chem 93:7409
González-Blanco C, Rodríguez LJ, Velázquez MM (1997) Langmuir 13:1938
Hou ZS, Li F, Wang HQ (2001) Colloid Polym Sci 279(1):8
Tamamusbi B, Watanabe N (1980) Colloid Polym Sci 258:174
Huang JS (1999) Langmuir 15:3718
Ikishima Y, Saito N, Arai M (1997) J Colloid Interface Sci 186:254
Hayes DG, Gulari E (1995) Langmuir 11:4695
Luan YX, Xu GY, Yuan SL, Xiao L, Zhang Z Q (2002) Langmuir 18:8700
Valstar A, Brown W, Almgren M (1999) Langmuir 15:2366
Xu GY, Zhang L, Mao HZ, Bao M, Lu Y (2001) Acta Phys Chim Sin 17:37 (in Chinese)
Meziani A, Touraud D, Zradba A, Clausse M, Kunz W (2000) J Mol Liquids 84:301
Filankembo A, André P, Lisiecki I, Petit C, Gulik-Krzywicki T, Ninham BW, Pileni MP (2000) Colloids Surf A: 174:221
César ATL, Wyn B, Mats A, Sílvia MBCosta (2000) Langmuir 16:465
Huruguen JP, Authier M, Greffe JL, Pileni PM (1991) Langmuir 7:243
Cassin G, Duda Y, Holovko M, Badiali JP, Pileni MP (1997) J Chem Phys 107:2683
Valstar A, Almgren M, Brown W (2000) Langmuir 16:922
Christoff M, Silveira N P, Samios D (2001) Langmuir 17:2885
Li ZM, Ye QM, Yang JP (1983) Macromol Comm 3:184 (in Chinese)
Kotlarchyk M (1982) J Phys Chem 86:3273
Liu K, Cruzan JD, Saykally RJ (1996) Science 271:929
Giammona G, Goffredi F, Liveri VT, Vassallo G (1992) J Colloid Interface Sci 154:411
Kitano H, Ichikawa K, Ide M, Fukuda M, Mizuno W (2001) Langmuir 17:1889
Hertz G (1975) In: Franks F (ed) Water—a comprehensive treatise, vol 3. Plenum Press, New York, chap 7
Xu GY, Zhang L, Yuang SL, Huang XR, Li GZ (2001) J Dispersion Sci Technol 22 (6):563
Aknowledgement.
The authors gratefully acknowledge financial support from National Natural Science Foundation (29973023) and National Microgravity Laboratory, Institute of Mechanics, CAS, Beijing, China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Luan, Y., Xu, G., Dai, G. et al. The interaction between poly(vinylpyrrolidone) and reversed micelles of water/AOT/n-heptane. Colloid Polym Sci 282, 110–118 (2003). https://doi.org/10.1007/s00396-003-0900-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-003-0900-7