Abstract.
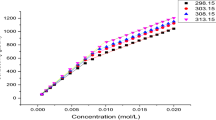
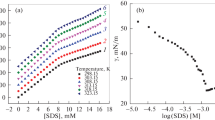
The values of the critical micelle concentration (cmc) and the degree of electrolytic micelle dissociation, a, for sodium dodecyl sulphate (SDS) as a function of the concentrations of the electrolytes added, NaCl, KCl, NaF, NaClO4, NH4ClO4, and Mg(ClO4)2, have been determined. The values of the SDS cmc have been shown to depend on the kind and concentration of the electrolyte cations. The electrolyte cations cause a decrease of the cmc in the following order: Na+<NH4 +<K+<Mg2+. Moreover, a depends on the kind and concentration of the electrolyte added. The electrolyte anions have a much smaller effect on the values of a than the cations. The anions enhance a in the following order: F–>ClO4 –>Cl–. The effect of different electrolyte cations on a is observed; moreover the values of a either increase or decrease with the electrolyte concentration. Other micellization parameters of SDS versus the concentration of the electrolytes added have been calculated.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Electronic Publication
Rights and permissions
About this article
Cite this article
Dutkiewicz, .E., Jakubowska, .A. Effect of electrolytes on the physicochemical behaviour of sodium dodecyl sulphate micelles. Colloid Polym Sci 280, 1009–1014 (2002). https://doi.org/10.1007/s00396-002-0723-y
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s00396-002-0723-y