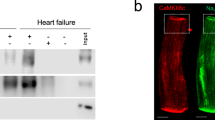
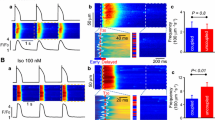
Abstract
Dysregulated intracellular Ca2+ handling involving altered Ca2+ release from intracellular stores via RyR channels underlies both arrhythmias and reduced function in heart failure (HF). Mechanisms linking RyR dysregulation and disease are not fully established. Studies in animals support a role for InsP3 receptor Ca2+ channels (InsP3R) in pathological alterations in cardiomyocyte Ca2+ handling but whether these findings translate to the divergent physiology of human cardiomyocytes during heart failure is not determined. Using electrophysiological and Ca2+ recordings in human ventricular cardiomyocytes, we uncovered that Ca2+ release via InsP3Rs facilitated Ca2+ release from RyR and induced arrhythmogenic delayed after depolarisations and action potentials. InsP3R–RyR crosstalk was particularly increased in HF at RyR clusters isolated from the T-tubular network. Reduced SERCA activity in HF further facilitated the action of InsP3. Nanoscale imaging revealed co-localisation of InsP3Rs with RyRs in the dyad, which was increased in HF, providing a mechanism for augmented Ca2+ channel crosstalk. Notably, arrhythmogenic activity dependent on InsP3Rs was increased in tissue wedges from failing hearts perfused with AngII to promote InsP3 generation. These data indicate a central role for InsP3R–RyR Ca2+ signalling crosstalk in the pro-arrhythmic action of GPCR agonists elevated in HF and the potential for their therapeutic targeting.






Similar content being viewed by others
Data availability
Data is available upon request.
References
Amoni M, Dries E, Ingelaere S, Vermoortele D, Roderick HL, Claus P, Willems R, Sipido KR (2021) Ventricular arrhythmias in ischemic cardiomyopathy-new avenues for mechanism-guided treatment. Cells 10:2629. https://doi.org/10.3390/cells10102629
Bare DJ, Kettlun CS, Liang M, Bers DM, Mignery GA (2005) Cardiac type 2 inositol 1,4,5-trisphosphate receptor: interaction and modulation by calcium/calmodulin-dependent protein kinase II. J Biol Chem 280:15912–15920. https://doi.org/10.1074/jbc.M414212200
Bers DM (2002) Cardiac excitation-contraction coupling. Nature 415:198–205. https://doi.org/10.1038/415198a
Bers DM, Pogwizd SM, Schlotthauer K (2002) Upregulated Na/Ca exchange is involved in both contractile dysfunction and arrhythmogenesis in heart failure. Basic Res Cardiol 97(Suppl 1):I36-42. https://doi.org/10.1007/s003950200027
Betzenhauser MJ, Wagner LE 2nd, Iwai M, Michikawa T, Mikoshiba K, Yule DI (2008) ATP modulation of Ca2+ release by type-2 and type-3 inositol (1, 4, 5)-triphosphate receptors. Differing ATP sensitivities and molecular determinants of action. J Biol Chem 283:21579–21587. https://doi.org/10.1074/jbc.M801680200
Beuckelmann DJ, Nabauer M, Erdmann E (1993) Alterations of K+ currents in isolated human ventricular myocytes from patients with terminal heart failure. Circ Res 73:379–385. https://doi.org/10.1161/01.res.73.2.379
Blanch ISJ, Egger M (2018) Obstruction of ventricular Ca(2+)—dependent arrhythmogenicity by inositol 1,4,5-trisphosphate-triggered sarcoplasmic reticulum Ca(2+) release. J Physiol 596:4323–4340. https://doi.org/10.1113/JP276319
Chung J, Tilūnaitė A, Ladd D, Hunt H, Soeller C, Crampin EJ, Johnson ST, Roderick HL, Rajagopal V (2022) IP3R activity increases propensity of RyR-mediated sparks by elevating dyadic [Ca2+]. Math Bio. https://doi.org/10.1016/j.mbs.2022.108923
De Smet MA, Lissoni A, Nezlobinsky T, Wang N, Dries E, Perez-Hernandez M, Lin X, Amoni M, Vervliet T, Witschas K, Rothenberg E, Bultynck G, Schulz R, Panfilov AV, Delmar M, Sipido KR, Leybaert L (2021) Cx43 hemichannel microdomain signaling at the intercalated disc enhances cardiac excitability. J Clin Invest. https://doi.org/10.1172/JCI137752
Demydenko K, Ekhteraei-Tousi S, Roderick HL (2022) Inositol 1,4,5-trisphosphate receptors in cardiomyocyte physiology and disease. Philos Trans R Soc Lond B Biol Sci 377:20210319. https://doi.org/10.1098/rstb.2021.0319
Demydenko K, Sipido KR, Roderick HL (2021) Ca2+ release via InsP3Rs enhances RyR recruitment during Ca2+ transients by increasing dyadic [Ca2+] in cardiomyocytes. J Cell Sci. https://doi.org/10.1242/jcs.258671
Dobrev D, Wehrens XH (2014) Role of RyR2 phosphorylation in heart failure and arrhythmias: Controversies around ryanodine receptor phosphorylation in cardiac disease. Circ Res 114:1311–1319. https://doi.org/10.1161/CIRCRESAHA.114.300568 (discussion 1319)
Domeier TL, Zima AV, Maxwell JT, Huke S, Mignery GA, Blatter LA (2008) IP3 receptor-dependent Ca2+ release modulates excitation-contraction coupling in rabbit ventricular myocytes. Am J Physiol Heart Circ Physiol 294:H596-604. https://doi.org/10.1152/ajpheart.01155.2007
Drawnel FM, Archer CR, Roderick HL (2013) The role of the paracrine/autocrine mediator endothelin-1 in regulation of cardiac contractility and growth. Br J Pharmacol 168:296–317. https://doi.org/10.1111/bph.2013.168.issue-1
Dries E, Amoni M, Vandenberk B, Johnson DM, Gilbert G, Nagaraju CK, Puertas RD, Abdesselem M, Santiago DJ, Roderick HL, Claus P, Willems R, Sipido KR (2020) Altered adrenergic response in myocytes bordering a chronic myocardial infarction underlies in vivo triggered activity and repolarization instability. J Physiol. https://doi.org/10.1113/JP278839
Dries E, Bito V, Lenaerts I, Antoons G, Sipido KR, Macquaide N (2013) Selective modulation of coupled ryanodine receptors during microdomain activation of calcium/calmodulin-dependent kinase II in the dyadic cleft. Circ Res 113:1242–1252. https://doi.org/10.1161/CIRCRESAHA.113.301896
Dries E, Santiago DJ, Gilbert G, Lenaerts I, Vandenberk B, Nagaraju CK, Johnson DM, Holemans P, Roderick HL, Macquaide N, Claus P, Sipido KR (2018) Hyperactive ryanodine receptors in human heart failure and ischaemic cardiomyopathy reside outside of couplons. Cardiovasc Res 114:1512–1524. https://doi.org/10.1093/cvr/cvy088
Fischer TH, Eiringhaus J, Dybkova N, Forster A, Herting J, Kleinwachter A, Ljubojevic S, Schmitto JD, Streckfuss-Bomeke K, Renner A, Gummert J, Hasenfuss G, Maier LS, Sossalla S (2014) Ca(2+) /calmodulin-dependent protein kinase II equally induces sarcoplasmic reticulum Ca(2+) leak in human ischaemic and dilated cardiomyopathy. Eur J Heart Fail 16:1292–1300. https://doi.org/10.1002/ejhf.163
Galice S, Xie Y, Yang Y, Sato D, Bers DM (2018) Size matters: ryanodine receptor cluster size affects arrhythmogenic sarcoplasmic reticulum calcium release. J Am Heart Assoc. https://doi.org/10.1161/JAHA.118.008724
Garg S, Narula J, Marelli C, Cesario D (2006) Role of angiotensin receptor blockers in the prevention and treatment of arrhythmias. Am J Cardiol 97:921–925. https://doi.org/10.1016/j.amjcard.2005.10.028
Gilbert G, Demydenko K, Dries E, Puertas RD, Jin X, Sipido K, Roderick HL (2020) calcium signaling in cardiomyocyte function. Cold Spring Harb Perspect Biol. https://doi.org/10.1101/cshperspect.a035428
Gomez AM, Valdivia HH, Cheng H, Lederer MR, Santana LF, Cannell MB, McCune SA, Altschuld RA, Lederer WJ (1997) Defective excitation-contraction coupling in experimental cardiac hypertrophy and heart failure. Science 276:800–806. https://doi.org/10.1126/science.276.5313.800
Harzheim D, Movassagh M, Foo RS, Ritter O, Tashfeen A, Conway SJ, Bootman MD, Roderick HL (2009) Increased InsP3Rs in the junctional sarcoplasmic reticulum augment Ca2+ transients and arrhythmias associated with cardiac hypertrophy. Proc Natl Acad Sci USA 106:11406–11411. https://doi.org/10.1073/pnas.0905485106
Hasenfuss G, Schillinger W, Lehnart SE, Preuss M, Pieske B, Maier LS, Prestle J, Minami K, Just H (1999) Relationship between Na+-Ca2+—exchanger protein levels and diastolic function of failing human myocardium. Circulation 99:641–648. https://doi.org/10.1161/01.cir.99.5.641
Hegyi B, Polonen RP, Hellgren KT, Ko CY, Ginsburg KS, Bossuyt J, Mercola M, Bers DM (2021) Cardiomyocyte Na(+) and Ca(2+) mishandling drives vicious cycle involving CaMKII, ROS, and ryanodine receptors. Basic Res Cardiol 116:58. https://doi.org/10.1007/s00395-021-00900-9
Heinzel FR, Bito V, Volders PG, Antoons G, Mubagwa K, Sipido KR (2002) Spatial and temporal inhomogeneities during Ca2+ release from the sarcoplasmic reticulum in pig ventricular myocytes. Circ Res 91:1023–1030. https://doi.org/10.1161/01.res.0000045940.67060.dd
Hohendanner F, Walther S, Maxwell JT, Kettlewell S, Awad S, Smith GL, Lonchyna VA, Blatter LA (2015) Inositol-1,4,5-trisphosphate induced Ca2+ release and excitation-contraction coupling in atrial myocytes from normal and failing hearts. J Physiol 593:1459–1477. https://doi.org/10.1113/jphysiol.2014.283226
Horn T, Ullrich ND, Egger M (2013) “Eventless” InsP3-dependent SR-Ca2+ release affecting atrial Ca2+ sparks. J Physiol 591:2103–2111. https://doi.org/10.1113/jphysiol.2012.247288
Jayasinghe I, Crossman D, Soeller C, Cannell M (2012) Comparison of the organization of T-tubules, sarcoplasmic reticulum and ryanodine receptors in rat and human ventricular myocardium. Clin Exp Pharmacol Physiol 39:469–476. https://doi.org/10.1111/j.1440-1681.2011.05578.x
Johnson DM, Antoons G (2018) Arrhythmogenic mechanisms in heart failure: linking beta-adrenergic stimulation, stretch, and calcium. Front Physiol 9:1453. https://doi.org/10.3389/fphys.2018.01453
Jung P, Seibertz F, Fakuade FE, Ignatyeva N, Sampathkumar S, Ritter M, Li H, Mason FE, Ebert A, Voigt N (2022) Increased cytosolic calcium buffering contributes to a cellular arrhythmogenic substrate in iPSC-cardiomyocytes from patients with dilated cardiomyopathy. Basic Res Cardiol 117:5. https://doi.org/10.1007/s00395-022-00912-z
Kockskamper J, Zima AV, Roderick HL, Pieske B, Blatter LA, Bootman MD (2008) Emerging roles of inositol 1,4,5-trisphosphate signaling in cardiac myocytes. J Mol Cell Cardiol 45:128–147. https://doi.org/10.1016/j.yjmcc.2008.05.014
Kolstad TR, van den Brink J, MacQuaide N, Lunde PK, Frisk M, Aronsen JM, Norden ES, Cataliotti A, Sjaastad I, Sejersted OM, Edwards AG, Lines GT, Louch WE (2018) Ryanodine receptor dispersion disrupts Ca(2+) release in failing cardiac myocytes. Elife. https://doi.org/10.7554/eLife.39427
Kranias EG, Hajjar RJ (2012) Modulation of cardiac contractility by the phospholamban/SERCA2a regulatome. Circ Res 110:1646–1660. https://doi.org/10.1161/CIRCRESAHA.111.259754
Kubalova Z, Terentyev D, Viatchenko-Karpinski S, Nishijima Y, Gyorke I, Terentyeva R, da Cunha DN, Sridhar A, Feldman DS, Hamlin RL, Carnes CA, Gyorke S (2005) Abnormal intrastore calcium signaling in chronic heart failure. Proc Natl Acad Sci USA 102:14104–14109. https://doi.org/10.1073/pnas.0504298102
Landstrom AP, Dobrev D, Wehrens XHT (2017) Calcium signaling and cardiac arrhythmias. Circ Res 120:1969–1993. https://doi.org/10.1161/CIRCRESAHA.117.310083
Ljubojevic S, Radulovic S, Leitinger G, Sedej S, Sacherer M, Holzer M, Winkler C, Pritz E, Mittler T, Schmidt A, Sereinigg M, Wakula P, Zissimopoulos S, Bisping E, Post H, Marsche G, Bossuyt J, Bers DM, Kockskamper J, Pieske B (2014) Early remodeling of perinuclear Ca2+ stores and nucleoplasmic Ca2+ signaling during the development of hypertrophy and heart failure. Circulation 130:244–255. https://doi.org/10.1161/CIRCULATIONAHA.114.008927
Lock JT, Alzayady KJ, Yule DI, Parker I (2018) All three IP3 receptor isoforms generate Ca(2+) puffs that display similar characteristics. Sci Signal. https://doi.org/10.1126/scisignal.aau0344
Louch WE, Bito V, Heinzel FR, Macianskiene R, Vanhaecke J, Flameng W, Mubagwa K, Sipido KR (2004) Reduced synchrony of Ca2+ release with loss of T-tubules-a comparison to Ca2+ release in human failing cardiomyocytes. Cardiovasc Res 62:63–73. https://doi.org/10.1016/j.cardiores.2003.12.031
Louch WE, Mork HK, Sexton J, Stromme TA, Laake P, Sjaastad I, Sejersted OM (2006) T-tubule disorganization and reduced synchrony of Ca2+ release in murine cardiomyocytes following myocardial infarction. J Physiol 574:519–533. https://doi.org/10.1113/jphysiol.2006.107227
Luo M, Anderson ME (2013) Mechanisms of altered Ca(2)(+) handling in heart failure. Circ Res 113:690–708. https://doi.org/10.1161/CIRCRESAHA.113.301651
Lyon AR, MacLeod KT, Zhang Y, Garcia E, Kanda GK, Lab MJ, Korchev YE, Harding SE, Gorelik J (2009) Loss of T-tubules and other changes to surface topography in ventricular myocytes from failing human and rat heart. Proc Natl Acad Sci U S A 106:6854–6859. https://doi.org/10.1073/pnas.0809777106
Mohler PJ, Davis JQ, Bennett V (2005) Ankyrin-B coordinates the Na/K ATPase, Na/Ca exchanger, and InsP3 receptor in a cardiac T-tubule/SR microdomain. PLoS Biol 3:e423. https://doi.org/10.1371/journal.pbio.0030423
Nakayama H, Bodi I, Maillet M, DeSantiago J, Domeier TL, Mikoshiba K, Lorenz JN, Blatter LA, Bers DM, Molkentin JD (2010) The IP3 receptor regulates cardiac hypertrophy in response to select stimuli. Circ Res 107:659–666. https://doi.org/10.1161/CIRCRESAHA.110.220038
Nattel S, Maguy A, Le Bouter S, Yeh YH (2007) Arrhythmogenic ion-channel remodeling in the heart: heart failure, myocardial infarction, and atrial fibrillation. Physiol Rev 87:425–456. https://doi.org/10.1152/physrev.00014.2006
Oikonomidis DL, Baltogiannis GG, Kolettis TM (2010) Do endothelin receptor antagonists have an antiarrhythmic potential during acute myocardial infarction? Evidence from experimental studies. J Interv Card Electrophysiol 28:157–165. https://doi.org/10.1007/s10840-010-9493-5
Orchard CH, Bryant SM, James AF (2013) Do t-tubules play a role in arrhythmogenesis in cardiac ventricular myocytes? J Physiol 591:4141–4147. https://doi.org/10.1113/jphysiol.2013.254540
Pieske B, Beyermann B, Breu V, Loffler BM, Schlotthauer K, Maier LS, Schmidt-Schweda S, Just H, Hasenfuss G (1999) Functional effects of endothelin and regulation of endothelin receptors in isolated human nonfailing and failing myocardium. Circulation 99:1802–1809. https://doi.org/10.1161/01.cir.99.14.1802
Pogwizd SM, Bers DM (2004) Cellular basis of triggered arrhythmias in heart failure. Trends Cardiovasc Med 14:61–66. https://doi.org/10.1016/j.tcm.2003.12.002
Priori SG, Chen SR (2011) Inherited dysfunction of sarcoplasmic reticulum Ca2+ handling and arrhythmogenesis. Circ Res 108:871–883. https://doi.org/10.1161/CIRCRESAHA.110.226845
Proven A, Roderick HL, Conway SJ, Berridge MJ, Horton JK, Capper SJ, Bootman MD (2006) Inositol 1,4,5-trisphosphate supports the arrhythmogenic action of endothelin-1 on ventricular cardiac myocytes. J Cell Sci 119:3363–3375. https://doi.org/10.1242/jcs.03073
Schendel T, Thul R, Sneyd J, Falcke M (2012) How does the ryanodine receptor in the ventricular myocyte wake up: by a single or by multiple open L-type Ca2+ channels? Eur Biophys J 41:27–39. https://doi.org/10.1007/s00249-011-0755-7
Schmitt N, Grunnet M, Olesen SP (2014) Cardiac potassium channel subtypes: new roles in repolarization and arrhythmia. Physiol Rev 94:609–653. https://doi.org/10.1152/physrev.00022.2013
Seidler NW, Jona I, Vegh M, Martonosi A (1989) Cyclopiazonic acid is a specific inhibitor of the Ca2+-ATPase of sarcoplasmic reticulum. J Biol Chem 264:17816–17823
Signore S, Sorrentino A, Ferreira-Martins J, Kannappan R, Shafaie M, Del Ben F, Isobe K, Arranto C, Wybieralska E, Webster A, Sanada F, Ogorek B, Zheng H, Liu X, Del Monte F, D’Alessandro DA, Wunimenghe O, Michler RE, Hosoda T, Goichberg P, Leri A, Kajstura J, Anversa P, Rota M (2013) Inositol 1, 4, 5-trisphosphate receptors and human left ventricular myocytes. Circulation 128:1286–1297. https://doi.org/10.1161/CIRCULATIONAHA.113.002764
Soltysinska E, Olesen SP, Christ T, Wettwer E, Varro A, Grunnet M, Jespersen T (2009) Transmural expression of ion channels and transporters in human nondiseased and end-stage failing hearts. Pflugers Arch 459:11–23. https://doi.org/10.1007/s00424-009-0718-3
Song LS, Sobie EA, McCulle S, Lederer WJ, Balke CW, Cheng H (2006) Orphaned ryanodine receptors in the failing heart. Proc Natl Acad Sci USA 103:4305–4310. https://doi.org/10.1073/pnas.0509324103
Szabo T, Geller L, Merkely B, Selmeci L, Juhasz-Nagy A, Solti F (2000) Investigating the dual nature of endothelin-1: ischemia or direct arrhythmogenic effect? Life Sci 66:2527–2541. https://doi.org/10.1016/s0024-3205(00)00587-7
Timmis A, Townsend N, Gale CP, Torbica A, Lettino M, Petersen SE, Mossialos EA, Maggioni AP, Kazakiewicz D, May HT, De Smedt D, Flather M, Zuhlke L, Beltrame JF, Huculeci R, Tavazzi L, Hindricks G, Bax J, Casadei B, Achenbach S, Wright L, Vardas P, European Society of C (2020) European society of cardiology: cardiovascular disease statistics 2019. Eur Heart J 41:12–85. https://doi.org/10.1093/eurheartj/ehz859
Wagner S, Maier LS, Bers DM (2015) Role of sodium and calcium dysregulation in tachyarrhythmias in sudden cardiac death. Circ Res 116:1956–1970. https://doi.org/10.1161/CIRCRESAHA.116.304678
Wullschleger M, Blanch J, Egger M (2017) Functional local crosstalk of inositol 1,4,5-trisphosphate receptor- and ryanodine receptor-dependent Ca2+ release in atrial cardiomyocytes. Cardiovasc Res 113:542–552. https://doi.org/10.1093/cvr/cvx020
Zhang YH, Hancox JC (2009) Regulation of cardiac Na+-Ca2+ exchanger activity by protein kinase phosphorylation–still a paradox? Cell Calcium 45:1–10. https://doi.org/10.1016/j.ceca.2008.05.005
Zima AV, Bovo E, Bers DM, Blatter LA (2010) Ca2+ spark-dependent and -independent sarcoplasmic reticulum Ca2+ leak in normal and failing rabbit ventricular myocytes. J Physiol 588:4743–4757. https://doi.org/10.1113/jphysiol.2010.197913
Acknowledgements
This work was supported by the Fund for Scientific Research-Flanders and KULeuven. Specifically, FWO projects G.0918.15 to KRS, G0C7319N to KRS and FR, FWO Project grant G08861N and FWO Odysseus Project 90663 to HLR, FWO postdoctoral fellowships to ED and GG and FWO PhD fellowship to MA. We also acknowledge support from the FWO for the STED Abberior microscope (Cell & tissue Imaging Cluster, CIC) (FWO Hercules funding I001918N to PVB, HLR et al.). We thank Annemie Biesmans for technical assistance, the transplant team of UZ Leuven for help in providing human explanted hearts, and Prof J Van Cleemput, head of the HF clinic for support in the research program. This work was supported by Fonds Wetenschappelijk Onderzoek (G.0617.09).
Author information
Authors and Affiliations
Contributions
Conceptualisation: HLR; Funding acquisition: HLR, KS; Study design: HLR, XJ, KS; Conducting experiments: XJ, RM, MA, GG, RD; Acquiring data: XJ, RM, MA, GG, RD; Analysing data: XJ, RM, MA, GG, HLR, TM, PVB, RD, AT; Providing reagents: DY, FR, CN; Writing the manuscript: XJ, HLR, KS; Writing review and editing: all authors.
Corresponding author
Ethics declarations
Conflict of interest
Dr. Rega has received research funding from Medtronic; and has been a consultant to AtriCure and LivaNova. All other authors declare no conflicts of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jin, X., Amoni, M., Gilbert, G. et al. InsP3R–RyR Ca2+ channel crosstalk facilitates arrhythmias in the failing human ventricle. Basic Res Cardiol 117, 60 (2022). https://doi.org/10.1007/s00395-022-00967-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-022-00967-y