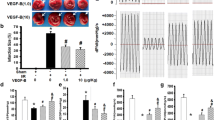
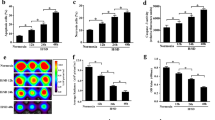
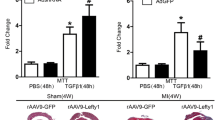
Abstract
Growth differentiation factor 11 (GDF11) is a member of the transforming growth factor beta 1 (TGF-β1) superfamily that reverses age-related cardiac hypertrophy, improves muscle regeneration and angiogenesis, and maintains progenitor cells in injured tissue. Recently, targeted myocardial delivery of the GDF11 gene in aged mice was found to reduce heart failure and enhance the proliferation of cardiac progenitor cells after myocardial ischemia–reperfusion (I–R). No investigations have as yet explored the cardioprotective effect of exogenous recombinant GDF11 in acute I–R injury, despite the convenience of its clinical application. We sought to determine whether exogenous recombinant GDF11 protects against acute myocardial I–R injury and investigate the underlying mechanism in Sprague–Dawley rats. We found that GDF11 reduced arrhythmia severity and successfully attenuated myocardial infarction; GDF11 also increased cardiac function after I–R, enhanced HO-1 expression and decreased oxidative damage. GDF11 activated the canonical TGF-β signaling pathway and inactivated the non-canonical pathways, ERK and JNK signaling pathways. Moreover, administration of GDF11 prior to reperfusion protected the heart from reperfusion damage. Notably, pretreatment with the activin-binding protein, follistatin (FST), inhibited the cardioprotective effects of GDF11 by blocking its activation of Smad2/3 signaling and its inactivation of detrimental TGF-β signaling. Our data suggest that exogenous GDF11 has cardioprotective effects and may have morphologic and functional recovery in the early stage of myocardial I–R injury. GDF11 may be an innovative therapeutic approach for reducing myocardial I–R injury.







Similar content being viewed by others
References
Arumugam TV, Okun E, Tang SC, Thundyil J, Taylor SM, Woodruff TM (2009) Toll-like receptors in ischemia-reperfusion injury. Shock 32:4–16. https://doi.org/10.1097/SHK.0b013e318193e333
Bolisetty S, Zarjou A, Agarwal A (2017) Heme oxygenase 1 as a therapeutic target in acute kidney injury. Am J Kidney Dis 69:531–545. https://doi.org/10.1053/j.ajkd.2016.10.037
Botker HE, Hausenloy D, Andreadou I, Antonucci S, Boengler K, Davidson SM, Deshwal S, Devaux Y, Di Lisa F, Di Sante M, Efentakis P, Femmino S, Garcia-Dorado D, Giricz Z, Ibanez B, Iliodromitis E, Kaludercic N, Kleinbongard P, Neuhauser M, Ovize M, Pagliaro P, Rahbek-Schmidt M, Ruiz-Meana M, Schluter KD, Schulz R, Skyschally A, Wilder C, Yellon DM, Ferdinandy P, Heusch G (2018) Practical guidelines for rigor and reproducibility in preclinical and clinical studies on cardioprotection. Basic Res Cardiol 113:39. https://doi.org/10.1007/s00395-018-0696-8
Buja LM (2005) Myocardial ischemia and reperfusion injury. Cardiovasc Pathol 14:170–175. https://doi.org/10.1016/j.carpath.2005.03.006
Cargnello M, Roux PP (2011) Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol Mol Biol Rev 75:50–83. https://doi.org/10.1128/MMBR.00031-10
Chen KM, Lee HH, Lu KH, Tseng YK, Hsu LS, Chou HL, Lai SC (2004) Association of matrix metalloproteinase-9 and Purkinje cell degeneration in mouse cerebellum caused by Angiostrongylus cantonensis. Int J Parasitol 34:1147–1156. https://doi.org/10.1016/j.ijpara.2004.07.004
Chen LL, Yin H, Huang J (2007) Inhibition of TGF-beta1 signaling by eNOS gene transfer improves ventricular remodeling after myocardial infarction through angiogenesis and reduction of apoptosis. Cardiovasc Pathol 16:221–230. https://doi.org/10.1016/j.carpath.2007.02.007
Chen Y, McMillan-Ward E, Kong J, Israels SJ, Gibson SB (2008) Oxidative stress induces autophagic cell death independent of apoptosis in transformed and cancer cells. Cell Death Differ 15:171–182. https://doi.org/10.1038/sj.cdd.4402233
Chen Y, Rothnie C, Spring D, Verrier E, Venardos K, Kaye D, Phillips DJ, Hedger MP, Smith JA (2014) Regulation and actions of activin A and follistatin in myocardial ischaemia-reperfusion injury. Cytokine 69:255–262. https://doi.org/10.1016/j.cyto.2014.06.017
Choo EH, Lee JH, Park EH, Park HE, Jung NC, Kim TH, Koh YS, Kim E, Seung KB, Park C, Hong KS, Kang K, Song JY, Seo HG, Lim DS, Chang K (2017) Infarcted myocardium-primed dendritic cells improve remodeling and cardiac function after myocardial infarction by modulating the regulatory T cell and macrophage polarization. Circulation 135:1444–1457. https://doi.org/10.1161/CIRCULATIONAHA.116.023106
Chow AK, Cena J, Schulz R (2007) Acute actions and novel targets of matrix metalloproteinases in the heart and vasculature. Br J Pharmacol 152:189–205. https://doi.org/10.1038/sj.bjp.0707344
Curtis MJ, Hancox JC, Farkas A, Wainwright CL, Stables CL, Saint DA, Clements-Jewery H, Lambiase PD, Billman GE, Janse MJ, Pugsley MK, Ng GA, Roden DM, Camm AJ, Walker MJ (2013) The Lambeth conventions (II): guidelines for the study of animal and human ventricular and supraventricular arrhythmias. Pharmacol Ther 139:213–248. https://doi.org/10.1016/j.pharmthera.2013.04.008
Deten A, Holzl A, Leicht M, Barth W, Zimmer HG (2001) Changes in extracellular matrix and in transforming growth factor beta isoforms after coronary artery ligation in rats. J Mol Cell Cardiol 33:1191–1207. https://doi.org/10.1006/jmcc.2001.1383
Djavaheri-Mergny M, Amelotti M, Mathieu J, Besancon F, Bauvy C, Souquere S, Pierron G, Codogno P (2006) NF-kappaB activation represses tumor necrosis factor-alpha-induced autophagy. J Biol Chem 281:30373–30382. https://doi.org/10.1074/jbc.M602097200
Du GQ, Shao ZB, Wu J, Yin WJ, Li SH, Wu J, Weisel RD, Tian JW, Li RK (2017) Targeted myocardial delivery of GDF11 gene rejuvenates the aged mouse heart and enhances myocardial regeneration after ischemia-reperfusion injury. Basic Res Cardiol 112:7. https://doi.org/10.1007/s00395-016-0593-y
Eltzschig HK, Eckle T (2011) Ischemia and reperfusion–from mechanism to translation. Nat Med 17:1391–1401. https://doi.org/10.1038/nm.2507
Folino A, Losano G, Rastaldo R (2013) Balance of nitric oxide and reactive oxygen species in myocardial reperfusion injury and protection. J Cardiovasc Pharmacol 62:567–575. https://doi.org/10.1097/FJC.0b013e3182a50c45
Hariharan N, Zhai P, Sadoshima J (2011) Oxidative stress stimulates autophagic flux during ischemia/reperfusion. Antioxid Redox Signal 14:2179–2190. https://doi.org/10.1089/ars.2010.3488
Harper SC, Johnson J, Borghetti G, Zhao H, Wang T, Wallner M, Kubo H, Feldsott EA, Yang Y, Joo Y, Gou X, Sabri AK, Gupta P, Myzithras M, Khalil A, Franti M, Houser SR (2018) GDF11 decreases pressure overload-induced hypertrophy, but can cause severe cachexia and premature death. Circ Res 123:1220–1231. https://doi.org/10.1161/CIRCRESAHA.118.312955
Hashemi M (2014) The study of pentoxifylline drug effects on renal apoptosis and BCL-2 gene expression changes following ischemic reperfusion injury in rat. Iran J Pharm Res 13:181–189
Hausenloy DJ, Botker HE, Engstrom T, Erlinge D, Heusch G, Ibanez B, Kloner RA, Ovize M, Yellon DM, Garcia-Dorado D (2017) Targeting reperfusion injury in patients with ST-segment elevation myocardial infarction: trials and tribulations. Eur Heart J 38:935–941. https://doi.org/10.1093/eurheartj/ehw145
Heusch G, Gersh BJ (2017) The pathophysiology of acute myocardial infarction and strategies of protection beyond reperfusion: a continual challenge. Eur Heart J 38:774–784. https://doi.org/10.1093/eurheartj/ehw224
Iles KE, Dickinson DA, Wigley AF, Welty NE, Blank V, Forman HJ (2005) HNE increases HO-1 through activation of the ERK pathway in pulmonary epithelial cells. Free Radic Biol Med 39:355–364. https://doi.org/10.1016/j.freeradbiomed.2005.03.026
Jazwa A, Stoszko M, Tomczyk M, Bukowska-Strakova K, Pichon C, Jozkowicz A, Dulak J (2015) HIF-regulated HO-1 gene transfer improves the post-ischemic limb recovery and diminishes TLR-triggered immune responses—effects modified by concomitant VEGF overexpression. Vascul Pharmacol 71:127–138. https://doi.org/10.1016/j.vph.2015.02.011
Jensen EC (2013) Quantitative analysis of histological staining and fluorescence using ImageJ. Anat Rec (Hoboken) 296:378–381. https://doi.org/10.1002/ar.22641
Kalogeris T, Baines CP, Krenz M, Korthuis RJ (2012) Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol 298:229–317. https://doi.org/10.1016/b978-0-12-394309-5.00006-7
Kalogeris T, Bao Y, Korthuis RJ (2014) Mitochondrial reactive oxygen species: a double edged sword in ischemia/reperfusion vs preconditioning. Redox Biol 2:702–714. https://doi.org/10.1016/j.redox.2014.05.006
Khalil AM, Dotimas H, Kahn J, Lamerdin JE, Hayes DB, Gupta P, Franti M (2016) Differential binding activity of TGF-beta family proteins to select TGF-beta receptors. J Pharmacol Exp Ther 358:423–430. https://doi.org/10.1124/jpet.116.232322
Li Q, Guo Y, Tan W, Stein AB, Dawn B, Wu WJ, Zhu X, Lu X, Xu X, Siddiqui T, Tiwari S, Bolli R (2006) Gene therapy with iNOS provides long-term protection against myocardial infarction without adverse functional consequences. Am J Physiol Heart Circ Physiol 290:H584–H589. https://doi.org/10.1152/ajpheart.00855.2005
Li S, Nie EH, Yin Y, Benowitz LI, Tung S, Vinters HV, Bahjat FR, Stenzel-Poore MP, Kawaguchi R, Coppola G, Carmichael ST (2015) GDF10 is a signal for axonal sprouting and functional recovery after stroke. Nat Neurosci 18:1737–1745. https://doi.org/10.1038/nn.4146
Lindsey ML, Bolli R, Canty JM Jr, Du XJ, Frangogiannis NG, Frantz S, Gourdie RG, Holmes JW, Jones SP, Kloner RA, Lefer DJ, Liao R, Murphy E, Ping P, Przyklenk K, Recchia FA, Schwartz Longacre L, Ripplinger CM, Van Eyk JE, Heusch G (2018) Guidelines for experimental models of myocardial ischemia and infarction. Am J Physiol Heart Circ Physiol 314:H812–H838. https://doi.org/10.1152/ajpheart.00335.2017
Loffredo FS, Steinhauser ML, Jay SM, Gannon J, Pancoast JR, Yalamanchi P, Sinha M, Dall’Osso C, Khong D, Shadrach JL, Miller CM, Singer BS, Stewart A, Psychogios N, Gerszten RE, Hartigan AJ, Kim MJ, Serwold T, Wagers AJ, Lee RT (2013) Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell 153:828–839. https://doi.org/10.1016/j.cell.2013.04.015
Ma S, Wang Y, Chen Y, Cao F (2015) The role of the autophagy in myocardial ischemia/reperfusion injury. Biochim Biophys Acta 1852:271–276. https://doi.org/10.1016/j.bbadis.2014.05.010
Marinkovic D, Zhang X, Yalcin S, Luciano JP, Brugnara C, Huber T, Ghaffari S (2007) Foxo3 is required for the regulation of oxidative stress in erythropoiesis. J Clin Invest 117:2133–2144. https://doi.org/10.1172/jci31807
Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H, Asano T, Levine B, Sadoshima J (2007) Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res 100:914–922. https://doi.org/10.1161/01.RES.0000261924.76669.36
Miyazono K (2000) Positive and negative regulation of TGF-beta signaling. J Cell Sci 113(Pt 7):1101–1109
Morciano G, Giorgi C, Bonora M, Punzetti S, Pavasini R, Wieckowski MR, Campo G, Pinton P (2015) Molecular identity of the mitochondrial permeability transition pore and its role in ischemia-reperfusion injury. J Mol Cell Cardiol 78:142–153. https://doi.org/10.1016/j.yjmcc.2014.08.015
Mozaffari MS, Liu JY, Abebe W, Baban B (2013) Mechanisms of load dependency of myocardial ischemia reperfusion injury. Am J Cardiovasc Dis 3:180–196
Opstad TB, Seljeflot I, Bohmer E, Arnesen H, Halvorsen S (2017) MMP-9 and its regulators TIMP-1 and EMMPRIN in patients with acute ST-elevation myocardial infarction: a NORDISTEMI substudy. Cardiology 139:17–24. https://doi.org/10.1159/000481684
Poillet-Perez L, Despouy G, Delage-Mourroux R, Boyer-Guittaut M (2015) Interplay between ROS and autophagy in cancer cells, from tumor initiation to cancer therapy. Redox Biol 4:184–192. https://doi.org/10.1016/j.redox.2014.12.003
Rabinowitz JD, White E (2010) Autophagy and metabolism. Science 330:1344–1348. https://doi.org/10.1126/science.1193497
Rochette L, Zeller M, Cottin Y, Vergely C (2015) Growth and differentiation factor 11 (GDF11): functions in the regulation of erythropoiesis and cardiac regeneration. Pharmacol Ther 156:26–33. https://doi.org/10.1016/j.pharmthera.2015.10.006
Saito T, Rodger IW, Shennib H, Hu F, Tayara L, Giaid A (2003) Cyclooxygenase-2 (COX-2) in acute myocardial infarction: cellular expression and use of selective COX-2 inhibitor. Can J Physiol Pharmacol 81:114–119. https://doi.org/10.1139/y03-023
Schofield ZV, Woodruff TM, Halai R, Wu MC, Cooper MA (2013) Neutrophils—a key component of ischemia-reperfusion injury. Shock 40:463–470. https://doi.org/10.1097/shk.0000000000000044
Su HH, Chu YC, Liao JM, Wang YH, Jan MS, Lin CW, Wu CY, Tseng CY, Yen JC, Huang SS (2017) Phellinus linteus mycelium alleviates myocardial ischemia-reperfusion injury through autophagic regulation. Front Pharmacol 8:175. https://doi.org/10.3389/fphar.2017.00175
Traylor A, Hock T, Hill-Kapturczak N (2007) Specificity protein 1 and Smad-dependent regulation of human heme oxygenase-1 gene by transforming growth factor-beta1 in renal epithelial cells. Am J Physiol Renal Physiol 293:F885–F894. https://doi.org/10.1152/ajprenal.00519.2006
Walker RG, Poggioli T, Katsimpardi L, Buchanan SM, Oh J, Wattrus S, Heidecker B, Fong YW, Rubin LL, Ganz P, Thompson TB, Wagers AJ, Lee RT (2016) Biochemistry and biology of GDF11 and myostatin: similarities, differences, and questions for future investigation. Circ Res 118:1125–1141. https://doi.org/10.1161/circresaha.116.308391 (discussion 1142)
Wang J, Hu X, Jiang H (2015) The Nrf-2/ARE-HO-1 axis: an important therapeutic approach for attenuating myocardial ischemia and reperfusion injury-induced cardiac remodeling. Int J Cardiol 184:263–264. https://doi.org/10.1016/j.ijcard.2015.02.073
Wang YH, Chen KM, Chiu PS, Lai SC, Su HH, Jan MS, Lin CW, Lu DY, Fu YT, Liao JM, Chang JT, Huang SS (2016) Lumbrokinase attenuates myocardial ischemia-reperfusion injury by inhibiting TLR4 signaling. J Mol Cell Cardiol 99:113–122. https://doi.org/10.1016/j.yjmcc.2016.08.004
Whelan RS, Kaplinskiy V, Kitsis RN (2010) Cell death in the pathogenesis of heart disease: mechanisms and significance. Annu Rev Physiol 72:19–44. https://doi.org/10.1146/annurev.physiol.010908.163111
WHO (2018) The top 10 causes of death. In: Media Centre World Health Organization. https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death
Yang Y, Yang Y, Wang X, Du J, Hou J, Feng J, Tian Y, He L, Li X, Pei H (2017) Does growth differentiation factor 11 protect against myocardial ischaemia/reperfusion injury? A hypothesis. J Int Med Res 45:1629–1635. https://doi.org/10.1177/0300060516658984
Acknowledgements
Cryostat preparation for frozen sections and upright fluorescence microscopy investigations were performed in the Instrument Center of Chung Shan Medical University, which is supported by Chung Shan Medical University and National Science Council, Ministry of Education, Taichung, Taiwan.
Funding
This work was supported by research grants from Taiwan’s Ministry of Science and Technology (MOST) 106-2320-B-040-005 and MOST 106-2314-B-350-003 given to S-SH and S-KT.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
All authors declare that they have no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Su, HH., Liao, JM., Wang, YH. et al. Exogenous GDF11 attenuates non-canonical TGF-β signaling to protect the heart from acute myocardial ischemia–reperfusion injury. Basic Res Cardiol 114, 20 (2019). https://doi.org/10.1007/s00395-019-0728-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-019-0728-z