Abstract
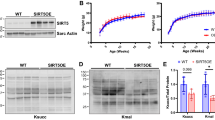
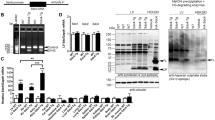
Dilated cardiomyopathy (DCM), the third most common cause of heart failure, is often associated with arrhythmias and sudden cardiac death if not controlled. The majority of DCM is of unknown etiology. Protein sialylation is altered in human DCM, with responsible mechanisms not yet described. Here we sought to investigate the impact of clinically relevant changes in sialylation on cardiac function using a novel model for altered glycoprotein sialylation that leads to DCM and to chronic stress-induced heart failure (HF), deletion of the sialyltransferase, ST3Gal4. We previously reported that 12- to 20-week-old ST3Gal4 −/− mice showed aberrant cardiac voltage-gated ion channel sialylation and gating that contribute to a pro-arrhythmogenic phenotype. Here, echocardiography supported by histology revealed modest dilated and thinner-walled left ventricles without increased fibrosis in ST3Gal4 −/− mice starting at 1 year of age. Cardiac calcineurin expression in younger (16–20 weeks old) ST3Gal4 −/− hearts was significantly reduced compared to WT. Transverse aortic constriction (TAC) was used as a chronic stressor on the younger mice to determine whether the ability to compensate against a pathologic insult is compromised in the ST3Gal4 −/− heart, as suggested by previous reports describing the functional implications of reduced cardiac calcineurin levels. TAC’d ST3Gal4 −/− mice presented with significantly reduced systolic function and ventricular dilation that deteriorated into congestive HF within 6 weeks post-surgery, while constricted WT hearts remained well-adapted throughout (ejection fraction, ST3Gal4 −/− = 34 ± 5.2 %; WT = 53.8 ± 7.4 %; p < 0.05). Thus, a novel, sialo-dependent model for DCM/HF is described in which clinically relevant reduced sialylation results in increased arrhythmogenicity and reduced cardiac calcineurin levels that precede cardiomyopathy and TAC-induced HF, suggesting a causal link among aberrant sialylation, chronic arrhythmia, reduced calcineurin levels, DCM in the absence of a pathologic stimulus, and stress-induced HF.







Similar content being viewed by others
References
Al-Owain M, Mohamed S, Kaya N, Zagal A, Matthijs G, Jaeken J (2010) A novel mutation and first report of dilated cardiomyopathy in ALG6-CDG (CDG-Ic): a case report. Orphanet J Rare Dis 5:7. doi:10.1186/1750-1172-5-7
Barrans JD, Allen PD, Stamatiou D, Dzau VJ, Liew CC (2002) Global gene expression profiling of end-stage dilated cardiomyopathy using a human cardiovascular-based cDNA microarray. Am J Pathol 160:2035–2043. doi:10.1016/S0002-9440(10)61153-4
Bennett E, Urcan MS, Tinkle SS, Koszowski AG, Levinson SR (1997) Contribution of sialic acid to the voltage dependence of sodium channel gating. A possible electrostatic mechanism. J Gen Physiol 109:327–343. doi:10.1085/jgp.109.3.327
Bennett ES (2002) Isoform-specific effects of sialic acid on voltage-dependent Na+ channel gating: functional sialic acids are localized to the S5–S6 loop of domain I. J Physiol 538:675–690. doi:10.1113/jphysiol.2001.013285
Bers DM (2000) Calcium fluxes involved in control of cardiac myocyte contraction. Circ Res 87:275–281. doi:10.1161/01.RES.87.4.275
Born GV, Palinski W (1985) Unusually high concentrations of sialic acids on the surface of vascular endothelia. Br J Exp Pathol 66:543–549
Brooks S, Hall DS (2012) Lectin histochemistry to detect altered glycosylation in cells and tissues. In: Dwek M, Brooks SA, Schumacher U (eds) Metastasis research protocols. Humana Press, Totowa, pp 31–50
Brooks SA, Leathem AJC, Schumacher U, Society RM (1997) Lectin histochemistry: a concise practical handbook. BIOS Scientific, Oxford
Bueno OF, Lips DJ, Kaiser RA, Wilkins BJ, Dai YS, Glascock BJ, Klevitsky R, Hewett TE, Kimball TR, Aronow BJ, Doevendans PA, Molkentin JD (2004) Calcineurin Abeta gene targeting predisposes the myocardium to acute ischemia-induced apoptosis and dysfunction. Circ Res 94:91–99. doi:10.1161/01.RES.0000107197.99679.77
Bueno OF, van Rooij E, Molkentin JD, Doevendans PA, De Windt LJ (2002) Calcineurin and hypertrophic heart disease: novel insights and remaining questions. Cardiovasc Res 53:806–821. doi:10.1016/S0008-6363(01)00493-X806-821
Bueno OF, Wilkins BJ, Tymitz KM, Glascock BJ, Kimball TF, Lorenz JN, Molkentin JD (2002) Impaired cardiac hypertrophic response in Calcineurin Abeta -deficient mice. Proc Natl Acad Sci USA 99:4586–4591. doi:10.1073/pnas.072647999
Bui AL, Horwich TB, Fonarow GC (2011) Epidemiology and risk profile of heart failure. Nat Rev Cardiol 8:30–41. doi:10.1038/nrcardio.2010.165
Camacho Londono JE, Tian Q, Hammer K, Schroder L, Camacho Londono J, Reil JC, He T, Oberhofer M, Mannebach S, Mathar I, Philipp SE, Tabellion W, Schweda F, Dietrich A, Kaestner L, Laufs U, Birnbaumer L, Flockerzi V, Freichel M, Lipp P (2015) A background Ca2 + entry pathway mediated by TRPC1/TRPC4 is critical for development of pathological cardiac remodelling. Eur Heart J 36:2257–2266. doi:10.1093/eurheartj/ehv250
Celeste FV, Vilboux T, Ciccone C, de Dios JK, Malicdan MC, Leoyklang P, McKew JC, Gahl WA, Carrillo-Carrasco N, Huizing M (2014) Mutation update for GNE gene variants associated with GNE myopathy. Hum Mutat 35:915–926. doi:10.1002/humu.22583
De Windt LJ, Lim HW, Bueno OF, Liang Q, Delling U, Braz JC, Glascock BJ, Kimball TF, del Monte F, Hajjar RJ, Molkentin JD (2001) Targeted inhibition of calcineurin attenuates cardiac hypertrophy in vivo. Proc Natl Acad Sci USA 98:3322–3327. doi:10.1073/pnas.031371998
Du D, Yang H, Yang H, Ednie A, Bennett E (2015) Statistical Metamodeling and Sequential Design of Computer Experiments to Model Glyco-altered Gating of Sodium Channels in Cardiac Myocytes. IEEE J Biomed Health Inform PP:1 doi:10.1109/JBHI.2015.2458791
Ednie AR, Bennett ES (2012) Modulation of voltage-gated ion channels by sialylation. Compr Physiol 2:1269–1301. doi:10.1002/cphy.c110044
Ednie AR, Bennett ES (2015) Reduced sialylation impacts ventricular repolarization by modulating specific K+ channel isoforms distinctly. J Biol Chem 290:2769–2783. doi:10.1074/jbc.M114.605139
Ednie AR, Horton KK, Wu J, Bennett ES (2013) Expression of the sialyltransferase, ST3Gal4, impacts cardiac voltage-gated sodium channel activity, refractory period and ventricular conduction. J Mol Cell Cardiol 59:117–127. doi:10.1016/j.yjmcc.2013.02.013
Ellies LG, Ditto D, Levy GG, Wahrenbrock M, Ginsburg D, Varki A, Le DT, Marth JD (2002) Sialyltransferase ST3Gal-IV operates as a dominant modifier of hemostasis by concealing asialoglycoprotein receptor ligands. Proc Natl Acad Sci USA 99:10042–10047. doi:10.1073/pnas.142005099
Elliott P, Andersson B, Arbustini E, Bilinska Z, Cecchi F, Charron P, Dubourg O, Kuhl U, Maisch B, McKenna WJ, Monserrat L, Pankuweit S, Rapezzi C, Seferovic P, Tavazzi L, Keren A (2008) Classification of the cardiomyopathies: a position statement from the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 29:270–276. doi:10.1093/eurheartj/ehm342
Felker GM, Thompson RE, Hare JM, Hruban RH, Clemetson DE, Howard DL, Baughman KL, Kasper EK (2000) Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med 342:1077–1084. doi:10.1056/NEJM200004133421502
Footitt EJ, Karimova A, Burch M, Yayeh T, Dupre T, Vuillaumier-Barrot S, Chantret I, Moore SE, Seta N, Grunewald S (2009) Cardiomyopathy in the congenital disorders of glycosylation (CDG): a case of late presentation and literature review. J Inherit Metab Dis 32(Suppl 1):S313–S319. doi:10.1007/s10545-009-1262-1
Francis GS, Desai MY (2008) Contractile reserve: are we beginning to understand it? JACC Cardiovasc Imaging 1:727–728. doi:10.1016/j.jcmg.2008.09.001
Gavillet B, Rougier JS, Domenighetti AA, Behar R, Boixel C, Ruchat P, Lehr HA, Pedrazzini T, Abriel H (2006) Cardiac sodium channel Nav1.5 is regulated by a multiprotein complex composed of syntrophins and dystrophin. Circ Res 99:407–414. doi:10.1161/01.RES.0000237466.13252.5e
Ge J, Sun A, Paajanen V, Wang S, Su C, Yang Z, Li Y, Wang S, Jia J, Wang K, Zou Y, Gao L, Wang K, Fan Z (2008) Molecular and clinical characterization of a novel SCN5A mutation associated with atrioventricular block and dilated cardiomyopathy. Circ Arrhythm Electrophysiol 1:83–92. doi:10.1161/CIRCEP.107.750752
Gehrmann J, Sohlbach K, Linnebank M, Bohles HJ, Buderus S, Kehl HG, Vogt J, Harms E, Marquardt T (2003) Cardiomyopathy in congenital disorders of glycosylation. Cardiol Young 13:345–351. doi:10.1017/S1047951103000702
Geisler C, Jarvis DL (2011) Effective glycoanalysis with Maackia amurensis lectins requires a clear understanding of their binding specificities. Glycobiology 21:988–993. doi:10.1093/glycob/cwr080
Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB, American Heart Association Statistics C, Stroke Statistics S (2013) Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation 127:e6–e245. doi:10.1161/CIR.0b013e31828124ad
Heineke J, Molkentin JD (2006) Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol 7:589–600. doi:10.1038/nrm1983
Heineke J, Wollert KC, Osinska H, Sargent MA, York AJ, Robbins J, Molkentin JD (2010) Calcineurin protects the heart in a murine model of dilated cardiomyopathy. J Mol Cell Cardiol 48:1080–1087. doi:10.1016/j.yjmcc.2009.10.012
Hu P, Zhang D, Swenson L, Chakrabarti G, Abel ED, Litwin SE (2003) Minimally invasive aortic banding in mice: effects of altered cardiomyocyte insulin signaling during pressure overload. Am J Physiol Heart Circ Physiol 285:H1261–H1269. doi:10.1152/ajpheart.00108.2003
Hwang JJ, Allen PD, Tseng GC, Lam CW, Fananapazir L, Dzau VJ, Liew CC (2002) Microarray gene expression profiles in dilated and hypertrophic cardiomyopathic end-stage heart failure. Physiol Genomics 10:31–44. doi:10.1152/physiolgenomics.00122.2001
Johnson D, Bennett ES (2006) Isoform-specific effects of the beta2 subunit on voltage-gated sodium channel gating. J Biol Chem 281:25875–25881. doi:10.1074/jbc.M605060200
Johnson D, Montpetit ML, Stocker PJ, Bennett ES (2004) The sialic acid component of the beta1 subunit modulates voltage-gated sodium channel function. J Biol Chem 279:44303–44310. doi:10.1074/jbc.M408900200
Knezevic A, Polasek O, Gornik O, Rudan I, Campbell H, Hayward C, Wright A, Kolcic I, O’Donoghue N, Bones J, Rudd PM, Lauc G (2009) Variability, heritability and environmental determinants of human plasma N-glycome. J Proteome Res 8:694–701. doi:10.1021/pr800737u
Kono M, Ohyama Y, Lee YC, Hamamoto T, Kojima N, Tsuji S (1997) Mouse beta-galactoside alpha 2,3-sialyltransferases: comparison of in vitro substrate specificities and tissue specific expression. Glycobiology 7:469–479. doi:10.1093/glycob/7.4.469
Kranz C, Basinger AA, Gucsavas-Calikoglu M, Sun L, Powell CM, Henderson FW, Aylsworth AS, Freeze HH (2007) Expanding spectrum of congenital disorder of glycosylation Ig (CDG-Ig): sibs with a unique skeletal dysplasia, hypogammaglobulinemia, cardiomyopathy, genital malformations, and early lethality. Am J Med Genet A 143A:1371–1378. doi:10.1002/ajmg.a.31791
Li HH, Kedar V, Zhang C, McDonough H, Arya R, Wang DZ, Patterson C (2004) Atrogin-1/muscle atrophy F-box inhibits calcineurin-dependent cardiac hypertrophy by participating in an SCF ubiquitin ligase complex. J Clin Invest 114:1058–1071. doi:10.1172/JCI22220
Mann SA, Castro ML, Ohanian M, Guo G, Zodgekar P, Sheu A, Stockhammer K, Thompson T, Playford D, Subbiah R, Kuchar D, Aggarwal A, Vandenberg JI, Fatkin D (2012) R222Q SCN5A mutation is associated with reversible ventricular ectopy and dilated cardiomyopathy. J Am Coll Cardiol 60:1566–1573. doi:10.1016/j.jacc.2012.05.050
McNair WP, Ku L, Taylor MR, Fain PR, Dao D, Wolfel E, Mestroni L, Familial Cardiomyopathy Registry Research G (2004) SCN5A mutation associated with dilated cardiomyopathy, conduction disorder, and arrhythmia. Circulation 110:2163–2167. doi:10.1161/01.CIR.0000144458.58660.BB
McNair WP, Sinagra G, Taylor MR, Di Lenarda A, Ferguson DA, Salcedo EE, Slavov D, Zhu X, Caldwell JH, Mestroni L, Familial Cardiomyopathy Registry Research G (2011) SCN5A mutations associate with arrhythmic dilated cardiomyopathy and commonly localize to the voltage-sensing mechanism. J Am Coll Cardiol 57:2160–2168. doi:10.1016/j.jacc.2010.09.084
Molkentin JD (2004) Calcineurin-NFAT signaling regulates the cardiac hypertrophic response in coordination with the MAPKs. Cardiovasc Res 63:467–475. doi:10.1016/j.cardiores.2004.01.021
Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, Olson EN (1998) A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell 93:215–228. doi:10.1016/S0092-8674(00)81573-1
Montpetit ML, Stocker PJ, Schwetz TA, Harper JM, Norring SA, Schaffer L, North SJ, Jang-Lee J, Gilmartin T, Head SR, Haslam SM, Dell A, Marth JD, Bennett ES (2009) Regulated and aberrant glycosylation modulate cardiac electrical signaling. Proc Natl Acad Sci USA 106:16517–16522. doi:10.1073/pnas.0905414106
Nakayama H, Wilkin BJ, Bodi I, Molkentin JD (2006) Calcineurin-dependent cardiomyopathy is activated by TRPC in the adult mouse heart. FASEB J: Off Public Fed Am Soc Exp Biol 20:1660–1670. doi:10.1096/fj.05-5560com
Nanka O, Peumans WJ, Van Damme EJ, Pfuller U, Valasek P, Halata Z, Schumacher U, Grim M (2001) Lectin histochemistry of microvascular endothelium in chick and quail musculature. Anat Embryol (Berl) 204:407–411. doi:10.1007/s004290100212
Nguyen TP, Wang DW, Rhodes TH, George AL Jr (2008) Divergent biophysical defects caused by mutant sodium channels in dilated cardiomyopathy with arrhythmia. Circ Res 102:364–371. doi:10.1161/CIRCRESAHA.107.164673
Ohtsubo K, Marth JD (2006) Glycosylation in cellular mechanisms of health and disease. Cell 126:855–867. doi:10.1016/j.cell.2006.08.019
Okumura T, Murohara T (2013) Contractile reserve in dilated cardiomyopathy. In: José Milei GA (ed) Cardiomyopathies. InTech, pp 47–59. doi:10.5772/55413
Ram R, Mickelsen DM, Theodoropoulos C, Blaxall BC (2011) New approaches in small animal echocardiography: imaging the sounds of silence. Am J Physiol Heart Circ Physiol 301:H1765–H1780. doi:10.1152/ajpheart.00559.2011
Schaeffer PJ, Desantiago J, Yang J, Flagg TP, Kovacs A, Weinheimer CJ, Courtois M, Leone TC, Nichols CG, Bers DM, Kelly DP (2009) Impaired contractile function and calcium handling in hearts of cardiac-specific calcineurin b1-deficient mice. Am J Physiol Heart Circ Physiol 297:H1263–H1273. doi:10.1152/ajpheart.00152.2009
Schwetz TA, Norring SA, Bennett ES (2010) N-glycans modulate K(v)1.5 gating but have no effect on K(v)1.4 gating. Biochim Biophys Acta 1798:367–375. doi:10.1016/j.bbamem.2009.11.018
Simantirakis EN, Koutalas EP, Vardas PE (2012) Arrhythmia-induced cardiomyopathies: the riddle of the chicken and the egg still unanswered? Europace 14:466–473. doi:10.1093/europace/eur348
Sperandio M, Frommhold D, Babushkina I, Ellies LG, Olson TS, Smith ML, Fritzsching B, Pauly E, Smith DF, Nobiling R, Linderkamp O, Marth JD, Ley K (2006) Alpha 2,3-sialyltransferase-IV is essential for L-selectin ligand function in inflammation. Eur J Immunol 36:3207–3215. doi:10.1002/eji.200636157
Stocker PJ, Bennett ES (2006) Differential sialylation modulates voltage-gated Na+ channel gating throughout the developing myocardium. J Gen Physiol 127:253–265. doi:10.1085/jgp.200509423
Takashima S (2008) Characterization of mouse sialyltransferase genes: their evolution and diversity. Biosci Biotechnol Biochem 72:1155–1167. doi:10.1271/bbb.80025
Tang M, Li J, Huang W, Su H, Liang Q, Tian Z, Horak KM, Molkentin JD, Wang X (2010) Proteasome functional insufficiency activates the calcineurin-NFAT pathway in cardiomyocytes and promotes maladaptive remodelling of stressed mouse hearts. Cardiovasc Res 88:424–433. doi:10.1093/cvr/cvq217
Towbin JA, Lorts A (2011) Arrhythmias and dilated cardiomyopathy common pathogenetic pathways? J Am Coll Cardiol 57:2169–2171. doi:10.1016/j.jacc.2010.11.061
Ufret-Vincenty CA, Baro DJ, Lederer WJ, Rockman HA, Quinones LE, Santana LF (2001) Role of sodium channel deglycosylation in the genesis of cardiac arrhythmias in heart failure. J Biol Chem 276:28197–28203. doi:10.1074/jbc.M102548200
Verma SK, Krishnamurthy P, Kishore R (2014) Transverse aortic constriction: a model to study heart failure in small animals. In: Ardehali H, Bolli R, Losordo DW (eds) Manual of research techniques in cardiovascular medicine. Wiley, Oxford, UK. doi:10.1002/9781118495148.ch20
Vinhas M, Araujo AC, Ribeiro S, Rosario LB, Belo JA (2013) Transthoracic echocardiography reference values in juvenile and adult 129/Sv mice. Cardiovasc Ultrasound 11:12. doi:10.1186/1476-7120-11-12
Wan E, Abrams J, Weinberg RL, Katchman AN, Bayne J, Zakharov SI, Yang L, Morrow JP, Garan H, Marx SO (2016) Aberrant sodium influx causes cardiomyopathy and atrial fibrillation in mice. J Clin Invest 126:112–122. doi:10.1172/JCI84669
Waszkiewicz N, Szajda SD, Zalewska A, Szulc A, Kepka A, Minarowska A, Wojewodzka-Zelezniakowicz M, Konarzewska B, Chojnowska S, Ladny JR, Zwierz K (2012) Alcohol abuse and glycoconjugate metabolism. Folia Histochem Cytobiol 50:1–11. doi:10.2478/18690
Watanabe H, Yang T, Stroud DM, Lowe JS, Harris L, Atack TC, Wang DW, Hipkens SB, Leake B, Hall L, Kupershmidt S, Chopra N, Magnuson MA, Tanabe N, Knollmann BC, George AL Jr, Roden DM (2011) Striking In vivo phenotype of a disease-associated human SCN5A mutation producing minimal changes in vitro. Circulation 124:1001–1011. doi:10.1161/CIRCULATIONAHA.110.987248
Wilkins BJ, Molkentin JD (2002) Calcineurin and cardiac hypertrophy: where have we been? Where are we going? J Physiol 541:1–8. doi:10.1113/jphysiol.2002.017129
Wolska BM (2009) Calcineurin and cardiac function: is more or less better for the heart? Am J Physiol Heart Circ Physiol 297:H1576–H1577. doi:10.1152/ajpheart.00833.2009
Wu AM, Wu JH, Tsai MS, Yang Z, Sharon N, Herp A (2007) Differential affinities of Erythrina cristagalli lectin (ECL) toward monosaccharides and polyvalent mammalian structural units. Glycoconj J 24:591–604. doi:10.1007/s10719-007-9063-y
Yung CK, Halperin VL, Tomaselli GF, Winslow RL (2004) Gene expression profiles in end-stage human idiopathic dilated cardiomyopathy: altered expression of apoptotic and cytoskeletal genes. Genomics 83:281–297. doi:10.1016/j.ygeno.2003.08.007
Zou Y, Hiroi Y, Uozumi H, Takimoto E, Toko H, Zhu W, Kudoh S, Mizukami M, Shimoyama M, Shibasaki F, Nagai R, Yazaki Y, Komuro I (2001) Calcineurin plays a critical role in the development of pressure overload-induced cardiac hypertrophy. Circulation 104:97–101. doi:10.1161/01.CIR.104.1.97
Acknowledgments
We would like to thank Dr. Jamey Marth for generously providing the ST3Gal4 +/− mouse strain. This work was supported, in part, by two Grants from the National Science Foundation [IOS-1146882 and CMMI-1266331]; an American Heart Association, Greater Southeast Affiliate Grant-In-Aid [14GRNT20450148]; an American Heart Association, Greater Southeast Affiliate Postdoctoral Fellowship [15POST25710010]; and a Grant from the National Heart, Lung, Blood Institute [1R01HL102171-03].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical standards
All animal studies have been approved by the appropriate ethics committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Specific national laws have been observed. The manuscript does not contain clinical studies or patient data.
Rights and permissions
About this article
Cite this article
Deng, W., Ednie, A.R., Qi, J. et al. Aberrant sialylation causes dilated cardiomyopathy and stress-induced heart failure. Basic Res Cardiol 111, 57 (2016). https://doi.org/10.1007/s00395-016-0574-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-016-0574-1