Abstract
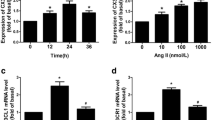
Chronic activation of angiotensin II (ANGII) and matrix metalloproteinase-2 (MMP-2) during hypertension contributes to increased aortic stiffness. We studied signalling mechanisms employed by ANGII in the regulation of latent (pro-) and active forms of MMP-2 in rat aortic endothelial and smooth muscle cells, along with isolated rat aorta. Using western blotting, we demonstrate that ANGII (1 µmol/L) significantly (P < 0.01) increases pro-MMP-2 protein expression after 8 h not only in endothelial and smooth muscle cells, but also in isolated rat aorta. We demonstrate that ANGII acts via AT1 receptor-activated cell-specific pathways. In endothelial cells, the JNK1/c-jun pathway is activated, whereas in smooth muscle cells, the JAK2/STAT3 pathway. Activation of JAK2/STAT3 pathway in response to ANGII was EGF receptor-dependent. Results obtained in cell culture are in agreement with the results obtained in isolated aorta. However, active MMP-2 was not found under cell culture conditions, whereas in isolated aorta, active MMP-2 was significantly (P < 0.05) increased after stimulation with ANGII, as detected by gelatine zymography. This increase of MMP-2 activity was not inhibited by blocking the pathways we identified to control pro-MMP-2 protein expression, but was abolished in the absence of endothelium. Our findings demonstrate that ANGII regulates pro-MMP-2 protein expression via cell-specific pathways in rat aorta. The endothelium may play an essential role in the activation of pro-MMP-2. These results may lead to new strategies for inhibiting MMP-2 expression and activity in distinct cell types of the aortic wall.










Similar content being viewed by others
References
Albaladejo P, Bouaziz H, Duriez M, Gohlke P, Levy BI, Safar ME, Benetos A (1994) Angiotensin converting enzyme inhibition prevents the increase in aortic collagen in rats. Hypertension 23:74–82. doi:10.1161/01.HYP.23.1.74
Arenas I, Xu Y, Lopez-Jaramillo P, Davidge ST (2003) Angiotensin II-induced MMP-2 release from endothelial cells is mediated by TNF-alpha. Am J Physiol Cell Physiol 286:779–784. doi:10.1152/ajpcell.00398.2003
Basalyga DM, Simionescu DT, Xiong W, Baxter BT, Starcher BC, Vyavahare NR (2004) Elastin degradation and calcification in an abdominal aorta injury model. Circulation 110:3480–3487. doi:10.1161/01.CIR.0000148367.08413.E9
Berk BC, Corson MA (1997) Angiotensin II signal transduction in vascular smooth muscle, role of tyrosine kinases. Circ Res 80:607–616. doi:10.1161/01.RES.80.5.607
Boutouyrie P, Bussy C, Hayoz D, Hengstler J, Dartois N, Laloux B, Brunner H, Laurent S (2000) Local pulse pressure and regression of arterial wall hypertrophy during long-term antihypertensive treatment. Circulation 101:2601–2606. doi:10.1161/01.CIR.101.22.2601
Browatzki M, Larsen D, Pfeiffer C, Gehrke SG, Schmidt J, Kranzhofer A, Katus H (2008) Angiotensin II stimulates matrix metalloproteinase secretion in human vascular smooth muscle cells via nuclear factor-kappaB and activator protein 1 in a redox-sensitive manner. J Vasc Res 42:415–423. doi:10.1016/j.regpep.2007.12.005
Bunkenburg B, van Amelsvoort T, Rogg H, Wood JM (1992) Receptor-mediated effects of angiotensin II on growth of vascular smooth muscle cells from spontaneously hypertensive rats. Hypertension 20:746–754. doi:10.1161/01.HYP.20.6.746
Chen LC, Noelken ME, Nagase H (1993) Disruption of the cysteine-75 and zinc ion coordination is not sufficient to activate the precursor of human matrix metalloproteinase 3 (stromelysin 1). Biochemistry 32:10289–10295
Cho A, Graves J, Reidy MA (2000) Mitogen-activated protein kinases mediate matrix metalloproteinase-9 expression in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 20:2527–2532. doi:10.1161/01.ATV.20.12.2527
Desk R, Williams L, Health K (2011) Matrix metalloproteinases in vascular remodelling and atherogenesis. Circ Res 90:251–262. doi:10.1161/hh0302.105345
Dzau VJ (1984) Vascular renin–angiotensin: a possible autocrine or paracrine system in control. J Cardiovasc Pharmacol 6:377–382
Frohlich ED, Sasaki O (1990) Dissociation of changes in cardiovascular mass and performance with angiotensin-converting enzyme inhibitors in Wistar-Kyoto and spontaneously hypertensive rats. J Am Coll Cardiol 16:1492–1499. doi:10.1016/0735-1097(90)90397-8
Gum R, Wang H, Lengyel E, Juarez J, Boyd D (1997) Regulation of 92 kDa type IV collagenase expression by the jun amino terminal kinase- and the extracellular signal-regulated kinase-dependent signalling cascades. Oncogene 14:1481–1493
Hanemaaijer R, Koolwijk P, Wil JA, Hinsbergh V (1993) Regulation of matrix metalloproteinase expression in human vein and microvascular endothelial cells. Biochem J 809:803–809
Ishi T, Asuwa N (2000) Collagen and elastin degradation by matrix metalloproteinases and tissue inhibitors of matrix metalloproteinase in aortic dissection. Hum Pathol 31:640–646. doi:10.1053/hupa.2000.7642
Ishiguro K, Hayashi K, Sasamura H, Sakamaki Y, Itoh H (2009) “Pulse” treatment with high-dose angiotensin blocker reverses renal arteriolar hypertrophy and regresses hypertension. Hypertension 53:83–89. doi:10.1161/HYPERTENSIONAHA.108.122721
Jiménes E, Pérez de la Blanca E, Urso L, González I, Salas J, Montiel M (2009) Angiotensin II induces MMP-2 activity via FAK/JNK pathway in human endothelial cells. BBRC 380:769–774. doi:10.1016/j.bbrc.2009.01.142
Lee Jin-Hee, Johnson Peter RA, Roth Michael, Hunt Nicholas H, Black Judith L (2001) ERK activation and mitogenesis in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 280:1019–1029
Keshet Y, Seger R (2010) The MAP kinase signalling cascades: a system of hundreds of components regulates a diverse array of physiological functions. Methods Mol Biol 661:3–38. doi:10.1007/978-1-60761-795-2_1
Knox JB, Sukhova GK, Whittemore AD, Libby P (1997) Evidence for altered balance between matrix metalloproteinases and their inhibitors in human aortic diseases. Circulation 95:205–212. doi:10.1161/01.CIR.95.1.205
Kroll K, Kelm MK, Burrig KF, Schrader J (1989) Transendothelial transport and metabolism of adenosine and inosine in the intact rat aorta. Circ Res 64:1147–1157. doi:10.1161/01.RES.64.6.1147
Lee YJ, Lee EB, Kwon YE, Lee JJ, Cho WS, Kim H, Song YW (2003) Effect of estrogen on the expression of matrix metalloproteinase (MMP)-1, MMP-3, and MMP-13 and tissue inhibitor of metalloproternase-1 in osteoarthritis chondrocytes. Rheumatol Int 23:282–288. doi:10.1007/s00296-003-0312-5
Liacini A, Sylvester J, Li WQ, Huang W, Dehnade F, Ahmad M, Zafarullaha M (2003) Induction of matrix metalloproteinase-13 gene expression by TNF-α is mediated by MAP kinases, AP-1, and NF-κB transcription factors in articular chondrocytes. Exp Cell Res 288:208–217. doi:10.1016/S0014-4827(03)00180-0
Makowski GS, Ramsby ML (2005) Autoactivation profiles of calcium-dependent matrix metalloproteinase-2 and -9 in inflammatory synovial fluid: effect of pyrophosphate and bisphosphonates. Clin Chem Acta 358:182–191. doi:10.1016/j.cccn.2005.03.012
Jamil Mayet, Stanton AV, Sinclair A-M, MacKay J, Shahi M, Foale RA, Nicolaides A, Poulter NR, Sever PS, McG. Thom SA, Hughes AD (1995) The effects of antihypertensive therapy on carotid vascular structure in man. Cardiovasc Res 30:147–152. doi:10.1016/S0008-6363(95)00026-7
Nagase H (1997) Activation mechanisms of matrix metalloproteinases. Biol Chem 387:151–160
Nagase H, Woessner JF (1999) Matrix metalloproteinases. J Biol Chem 274:21491–21494. doi:10.1074/jbc.274.31.21491
Naftilan AJ, Pratt RE, Dzau VJ (1989) Induction of platelet-derived growth factor A-chain and c-myc gene expressions by angiotensin II in cultured rat vascular smooth muscle cells. J Clin Invest 83:1419–1424
Naito T, Masaki T, Nikolic-Paterson DJ, Tanji C, Yorioka N, Kohno N (2004) Angiotensin II induces thrombospondin-1 production in human mesangial cells via p38 MAPK and JNK: a mechanism for activation of latent TGF-beta1. Am J Physiol Renal Physiol 286:278–287. doi:10.1152/ajprenal.00139.2003
Fabunmi Rosalind P, Baker Andrew H, Murray Edward J, Booth Robert FG, Newby Andrew C (1996) Divergent regulation by growth factors and cytokines of 95 kDa and 72 kDa gelatinases and tissue inhibitors of metalloproteinases-1, -2 and -3 in rabbit aortic smooth muscle cells. Biochem J 315:335–342
Safar ME, Girerd X, Laurent S (1996) Structural changes of large conduit arteries in hypertension. J Hypertens 14:545–555
Safar ME, Levy BI, Struijker-Boudier H (2003) Current perspectives on arterial stiffness and pulse pressure in hypertension and cardiovascular diseases. Circulation 107:2864–2869. doi:10.1161/01.CIR.0000069826.36125.B4
Stetler-Stevenson WG (1994) Progelatinase A activation during tumor cell invasion. Invas Metastas 14:259–268
Strongin AY, Collier I, Bannikov G, Marmer BL, Grant GA, Goldberg GI (1995) Mechanism of cell surface activation of 72-kDa type IV collagenase: isolation of the activated form of the membrane metalloprotease. J Biol Chem 270:5331–5338. doi:10.1074/jbc.270.10.5331
Toth M, Fridman R (2001) Assessment of gelatinases (MMP-2 and MMP-9) by gelatine zymography. In: Brooks SA, Schumacher U (eds) Metastasis Research Protocols, vol 1, 1st edn. Springer, Totowa, pp 163–173
Tyagi SC, Matsubara L, Weber KT (1993) Direct extraction and estimation of collagenase(s) activity by zymography in microquantities of rat myocardium and uterus. Clin Biochem 26:191–198. doi:10.1016/0009-9120(93)90025-2
Van Wart HE, Birkedal-Hansen H (1990) The cysteine switch: a principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene family. Proc Natl Acad Sci USA 87:5578–5582. doi:10.1073/pnas.87.14.5578
Visse R, Nagase H (2003) Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res 92:827–839. doi:10.1161/01.RES.0000070112.80711.3D
Yang X, Zhu MJ, Sreejayan N, Ren J, Du M (2005) Angiotensin II promotes smooth muscle cell proliferation and migration through release of heparin-binding epidermal growth factor and activation of EGF-receptor pathway. Mol Cell 20:263–270
Zatschler B, Dieterich P, Müller B, Kasper M, Rauen U, Deussen A (2009) Improved vessel preservation after 4 days of cold storage: experimental study in rat arteries. J Vasc Surg 50:397–406. doi:10.1016/j.jvs.2009.04.064
Acknowledgments
This work was supported by a grant-in-aid from the Federal Ministry of Education and Research of Germany (BMBF) (Grant: 0315473A).
Conflict of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kopaliani, I., Martin, M., Zatschler, B. et al. Cell-specific and endothelium-dependent regulations of matrix metalloproteinase-2 in rat aorta. Basic Res Cardiol 109, 419 (2014). https://doi.org/10.1007/s00395-014-0419-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-014-0419-8