Abstract
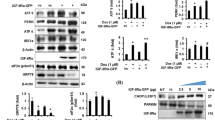
Preventing cyclophilin D (cypD) translocation to the inner mitochondrial membrane can limit lethal reperfusion injury through the inhibition of the opening of the mitochondrial permeability transition pore. Inhibition or loss of function of cypD may also result into an endoplasmic reticulum (ER) stress that has been shown to alter cell survival. We therefore questioned whether ER stress might play a role in the protection induced by CypD deficiency or inhibition. CypD-KO and NIM811 (a CypD inhibitor)-treated mice were subjected to a prolonged ischemia–reperfusion (I/R). Area at risk and infarct size was measured using blue dye and triphenyltetrazolium chloride staining. ER stress markers were measured in the hearts during the reperfusion phase. As expected, cypD-KO mice exhibited a decreased infarct size when compared to wild-type mice (8 ± 1 vs. 20 ± 4 % of left ventricular weight; p < 0.01). CypD-deficient mice displayed an increased expression of ER stress proteins such as eukaryotic initiation factor 2α (eIF2α) or glucose regulated protein 78 (Grp78 or Bip). The ER stress inhibitor TUDCA prevented the infarct size reduction afforded by the loss of cypD function (mean infarct size averaged 21 ± 4 % of LV weight, p < 0.01 vs. cypD-KO). Similar results were obtained when NIM811, an analog of cyclosporine A, was used to pharmacologically (instead of genetically) inhibit cypD function. This study suggests that the ER stress induced by the inhibition of cypD function plays a key role in protecting the heart against lethal ischemia–reperfusion injury.




Similar content being viewed by others
References
Argaud L, Gateau-Roesch O, Muntean D, Chalabreysse L, Loufouat J, Robert D, Ovize M (2005) Specific inhibition of the mitochondrial permeability transition prevents lethal reperfusion injury. J Mol Cell Cardiol 38:367–374. pii: S0022-2828(04)00397-9
Argaud L, Loufouat J, Gateau-Roesch O, Gomez L, Robert D, Ovize M (2008) Persistent inhibition of mitochondrial permeability transition by preconditioning during the first hours of reperfusion. Shock 30:552–556. doi:10.1097/SHK.0b013e31816a1c1c
Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD (2005) Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 434:658–662. doi:10.1038/nature03434
Belaidi E, Beguin PC, Levy P, Ribuot C, Godin-Ribuot D (2008) Prevention of HIF-1 activation and iNOS gene targeting by low-dose cadmium results in loss of myocardial hypoxic preconditioning in the rat. Am J Physiol Heart Circ Physiol 294:H901–H908. doi:00715.2007
Ben Mosbah I, Alfany-Fernandez I, Martel C, Zaouali MA, Bintanel-Morcillo M, Rimola A, Rodes J, Brenner C, Rosello-Catafau J, Peralta C (2010) Endoplasmic reticulum stress inhibition protects steatotic and non-steatotic livers in partial hepatectomy under ischemia-reperfusion. Cell Death Dis 1:e52. doi:10.1038/cddis.2010.29
Bi M, Naczki C, Koritzinsky M, Fels D, Blais J, Hu N, Harding H, Novoa I, Varia M, Raleigh J, Scheuner D, Kaufman RJ, Bell J, Ron D, Wouters BG, Koumenis C (2005) ER stress-regulated translation increases tolerance to extreme hypoxia and promotes tumor growth. EMBO J 24:3470–3481. doi:10.1038/sj.emboj.7600777
Boengler K, Hilfiker-Kleiner D, Heusch G, Schulz R (2010) Inhibition of permeability transition pore opening by mitochondrial STAT3 and its role in myocardial ischemia/reperfusion. Basic Res Cardiol 105:771–785. doi:10.1007/s00395-010-0124-1
Ceylan-Isik AF, Sreejayan N, Ren J (2011) Endoplasmic reticulum chaperon tauroursodeoxycholic acid alleviates obesity-induced myocardial contractile dysfunction. J Mol Cell Cardiol 50:107–116. doi:10.1016/j.yjmcc.2010.10.023
Cho TH, Aguettaz P, Campuzano Larrea O, Charriaut-Marlangue C, Riou A, Berthezene Y, Nighoghossian N, Ovize M, Wiart M, Chauveau F (2012) Pre- and post-treatment with cyclosporine A in a rat model of transient focal cerebral ischaemia with multimodal MRI screening. Int J Stroke. doi:10.1111/j.1747-4949.2012.00849.x
Crompton M (1999) The mitochondrial permeability transition pore and its role in cell death. Biochem J 341(Pt 2):233–249
Deniaud A, Sharaf el dein O, Maillier E, Poncet D, Kroemer G, Lemaire C, Brenner C (2008) Endoplasmic reticulum stress induces calcium-dependent permeability transition, mitochondrial outer membrane permeabilization and apoptosis. Oncogene 27:285–299 doi:10.1038/sj.onc.1210638
Devalaraja-Narashimha K, Diener AM, Padanilam BJ Cyclophilin D deficiency prevents diet-induced obesity in mice. FEBS Lett 585:677–682. pii: S0014-5793(11)00061-5
Di Lisa F, Menabo R, Canton M, Barile M, Bernardi P (2001) Opening of the mitochondrial permeability transition pore causes depletion of mitochondrial and cytosolic NAD+ and is a causative event in the death of myocytes in postischemic reperfusion of the heart. J Biol Chem 276:2571–2575. doi:10.1074/jbc.M006825200
Elrod JW, Wong R, Mishra S, Vagnozzi RJ, Sakthievel B, Goonasekera SA, Karch J, Gabel S, Farber J, Force T, Brown JH, Murphy E, Molkentin JD Cyclophilin D controls mitochondrial pore-dependent Ca(2+) exchange, metabolic flexibility, and propensity for heart failure in mice. J Clin Invest 120:3680–3687. doi:10.1172/JCI43171
Gao X, Fu L, Xiao M, Xu C, Sun L, Zhang T, Zheng F, Mei C (2012) The nephroprotective effect of tauroursodeoxycholic acid on ischaemia/reperfusion-induced acute kidney injury by inhibiting endoplasmic reticulum stress. Basic Clin Pharmacol Toxicol 111:14–23. doi:10.1111/j.1742-7843.2011.00854.x
Giorgi C, De Stefani D, Bononi A, Rizzuto R, Pinton P (2009) Structural and functional link between the mitochondrial network and the endoplasmic reticulum. Int J Biochem Cell B 41:1817–1827. doi:10.1016/j.biocel.2009.04.010
Glembotski CC (2007) Endoplasmic reticulum stress in the heart. Circ Res 101:975–984. doi:10.1161/CIRCRESAHA.107.161273
Gomez L, Thibault H, Gharib A, Dumont JM, Vuagniaux G, Scalfaro P, Derumeaux G, Ovize M (2007) Inhibition of mitochondrial permeability transition improves functional recovery and reduces mortality following acute myocardial infarction in mice. Am J Physiol Heart Circ Physiol 293:H1654–H1661. doi:10.1152/ajpheart.01378.2006
Groenendyk J, Sreenivasaiah PK, Kim do H, Agellon LB, Michalak M (2010) Biology of endoplasmic reticulum stress in the heart. Circ Res 107:1185–1197. doi:10.1161/CIRCRESAHA.110.227033
Halestrap AP, McStay GP, Clarke SJ (2002) The permeability transition pore complex: another view. Biochimie 84:153–166. pii: S0300908402013755
Hausenloy DJ, Yellon DM, Mani-Babu S, Duchen MR (2004) Preconditioning protects by inhibiting the mitochondrial permeability transition. Am J Physiol Heart Circ Physiol 287:H841–H849. doi:10.1152/ajpheart.00678.2003
Hetz CA (2007) ER stress signaling and the BCL-2 family of proteins: from adaptation to irreversible cellular damage. Antiox Redox Sign 9:2345–2355. doi:10.1089/ars.2007.1793
Heusch G, Boengler K, Schulz R (2010) Inhibition of mitochondrial permeability transition pore opening: the Holy Grail of cardioprotection. Basic Res Cardiol 105:151–154. doi:10.1007/s00395-009-0080-9
Heusch G, Musiolik J, Gedik N, Skyschally A (2011) Mitochondrial STAT3 activation and cardioprotection by ischemic postconditioning in pigs with regional myocardial ischemia/reperfusion. Circ Res 109:1302–1308. doi:10.1161/CIRCRESAHA.111.255604
Hom JR, Gewandter JS, Michael L, Sheu SS, Yoon Y (2007) Thapsigargin induces biphasic fragmentation of mitochondria through calcium-mediated mitochondrial fission and apoptosis. J Cell Physiol 212:498–508. doi:10.1002/jcp.21051
Kudo T, Kanemoto S, Hara H, Morimoto N, Morihara T, Kimura R, Tabira T, Imaizumi K, Takeda M (2008) A molecular chaperone inducer protects neurons from ER stress. Cell Death Differ 15:364–375. doi:10.1038/sj.cdd.4402276
Lim SY, Davidson SM, Hausenloy DJ, Yellon DM (2007) Preconditioning and postconditioning: the essential role of the mitochondrial permeability transition pore. Cardiovasc Res 75:530–535. pii: S0008-6363(07)00197-6
Luvisetto S, Basso E, Petronilli V, Bernardi P, Forte M (2008) Enhancement of anxiety, facilitation of avoidance behavior, and occurrence of adult-onset obesity in mice lacking mitochondrial cyclophilin D. Neuroscience 155:585–596. pii: S0306-4522(08)00961-5
Martindale JJ, Fernandez R, Thuerauf D, Whittaker R, Gude N, Sussman MA, Glembotski CC (2006) Endoplasmic reticulum stress gene induction and protection from ischemia/reperfusion injury in the hearts of transgenic mice with a tamoxifen-regulated form of ATF6. Circ Res 98:1186–1193. doi:10.1074/jbc.M109.018036
Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y (2005) Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature 434:652–658. doi:10.1038/nature03317
Nazareth W, Yafei N, Crompton M (1991) Inhibition of anoxia-induced injury in heart myocytes by cyclosporin A. J Mol Cell Cardiol 23:1351–1354
Nickson P, Toth A, Erhardt P (2007) PUMA is critical for neonatal cardiomyocyte apoptosis induced by endoplasmic reticulum stress. Cardiovascular Res 73:48–56. doi:10.1016/j.cardiores.2006.10.001
Oida Y, Izuta H, Oyagi A, Shimazawa M, Kudo T, Imaizumi K, Hara H (2008) Induction of BiP, an ER-resident protein, prevents the neuronal death induced by transient forebrain ischemia in Gerbil. Brain Res 1208:217–224. doi:10.1016/j.brainres.2008.02.068
Okada K, Minamino T, Tsukamoto Y, Liao Y, Tsukamoto O, Takashima S, Hirata A, Fujita M, Nagamachi Y, Nakatani T, Yutani C, Ozawa K, Ogawa S, Tomoike H, Hori M, Kitakaze M (2004) Prolonged endoplasmic reticulum stress in hypertrophic and failing heart after aortic constriction: possible contribution of endoplasmic reticulum stress to cardiac myocyte apoptosis. Circulation 110:705–712. doi:10.1161/01.CIR.0000137836.95625.D4
Ono T, Nagasue N, Kohno H, Uchida M, Takemoto Y, Dhar DK, Nakamura T (1995) Effect of tauroursodeoxycholic acid on bile flow and calcium excretion in ischemia-reperfusion injury of rat livers. J Hepatol 23:582–590
Ouyang YBXL, Emery JF, Lee AS, Giffard RG (2011) Overexpressing GRP78 influences Ca2+ handling and function of mitochondria in astrocytes after ischemia-like stress. Mitochondrion 11:279–286. doi:10.1016/j.mito.2010.10.007
Penna C, Mancardi D, Raimondo S, Geuna S, Pagliaro P (2008) The paradigm of postconditioning to protect the heart. J Cell Mol Med 12:435–458. doi:10.1111/j.1582-4934.2007.00210.x
Petrovski G, Das S, Juhasz B, Kertesz A, Tosaki A, Das DK (2011) Cardioprotection by endoplasmic reticulum stress-induced autophagy. Antioxid Redox Signal 14:2191–2200. doi:10.1089/ars.2010.3486
Pino SC, O’Sullivan-Murphy B, Lidstone EA, Yang C, Lipson KL, Jurczyk A, diIorio P, Brehm MA, Mordes JP, Greiner DL, Rossini AA, Bortell R (2009) CHOP mediates endoplasmic reticulum stress-induced apoptosis in Gimap5-deficient T cells. PLoS ONE 4:e5468. doi:10.1371/journal.pone.0005468
Piot C, Croisille P, Staat P, Thibault H, Rioufol G, Mewton N, Elbelghiti R, Cung TT, Bonnefoy E, Angoulvant D, Macia C, Raczka F, Sportouch C, Gahide G, Finet G, Andre-Fouet X, Revel D, Kirkorian G, Monassier JP, Derumeaux G, Ovize M (2008) Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N Engl J Med 359:473–481. doi:10.1056/NEJMoa071142
Prachasilchai W, Sonoda H, Yokota-Ikeda N, Ito K, Kudo T, Imaizumi K, Ikeda M (2009) The protective effect of a newly developed molecular chaperone-inducer against mouse ischemic acute kidney injury. J Pharmacol Sci 109:311–314
Rasola A, Bernardi P (2007) The mitochondrial permeability transition pore and its involvement in cell death and in disease pathogenesis. Apoptosis 12:815–833. doi:10.1007/s10495-007-0723-y
Rieusset J, Fauconnier J, Paillard M, Belaidi E, Tubbs E, Chauvin MA, Durand A, Bravard A, Teixeira G, Bartosch B, Michelet M, Theurey P, Vial G, Demion M, Blond E, Zoulim F, Gomez L, Vidal H, Lacampagne A, Ovize M (2012) Disruption of cyclophilin D-mediated calcium transfer from the ER to mitochondria contributes to hepatic ER stress and insulin resistance. Hepatology. doi:10.1002/hep.26189
Rodrigues CM, Spellman SR, Sola S, Grande AW, Linehan-Stieers C, Low WC, Steer CJ (2002) Neuroprotection by a bile acid in an acute stroke model in the rat. J Cereb Blood Flow Metab 22:463–471
Ruiz-Meana M, Inserte J, Fernandez-Sanz C, Hernando V, Miro-Casas E, Barba I, Garcia-Dorado D (2011) The role of mitochondrial permeability transition in reperfusion-induced cardiomyocyte death depends on the duration of ischemia. Basic Res Cardiol 106:1259–1268. doi:10.1007/s00395-011-0225-5
Schinzel AC, Takeuchi O, Huang Z, Fisher JK, Zhou Z, Rubens J, Hetz C, Danial NN, Moskowitz MA, Korsmeyer SJ (2005) Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. Proc Natl Acad Sci USA 102:12005–12010
Schroder M (2008) Endoplasmic reticulum stress responses. Cell Mol Life Sci 65:862–894. doi:10.1007/s00018-007-7383-5
Scorrano L, Oakes SA, Opferman JT, Cheng EH, Sorcinelli MD, Pozzan T, Korsmeyer SJ (2003) BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science 300:135–139. doi:10.1126/science.1081208
Shintani-Ishida K, Nakajima M, Uemura K, Yoshida K (2006) Ischemic preconditioning protects cardiomyocytes against ischemic injury by inducing GRP78. Biochem Biophys Res Commun 345:1600–1605. doi:10.1016/j.bbrc.2006.05.077
Skyschally A, Schulz R, Heusch G (2010) Cyclosporine A at reperfusion reduces infarct size in pigs. Cardiovasc Drugs Ther 24:85–87. doi:10.1007/s10557-010-6219-y
Thuerauf DJ, Marcinko M, Gude N, Rubio M, Sussman MA, Glembotski CC (2006) Activation of the unfolded protein response in infarcted mouse heart and hypoxic cultured cardiac myocytes. Circ Res 99:275–282. doi:10.1161/01.RES.0000233317.70421.03
Toth A, Nickson P, Mandl A, Bannister ML, Toth K, Erhardt P (2007) Endoplasmic reticulum stress as a novel therapeutic target in heart diseases. Cardiovasc Hematol Disord Drug Targets 7:205–218
Travis DL, Fabia R, Netto GG, Husberg BS, Goldstein RM, Klintmalm GB, Levy MF (1998) Protection by cyclosporine A against normothermic liver ischemia-reperfusion in pigs. J Surg Res 75:116–126. doi:10.1006/jsre.1998.5297
Truettner JS, Hu K, Liu CL, Dietrich WD, Hu B (2009) Subcellular stress response and induction of molecular chaperones and folding proteins after transient global ischemia in rats. Brain Res 1249:9–18. doi:10.1016/j.brainres.2008.10.032
Vivaldi MT, Kloner RA, Schoen FJ (1985) Triphenyltetrazolium staining of irreversible ischemic injury following coronary artery occlusion in rats. Am J Pathol 121:522–530
Woodfield K, Ruck A, Brdiczka D, Halestrap AP (1998) Direct demonstration of a specific interaction between cyclophilin-D and the adenine nucleotide translocase confirms their role in the mitochondrial permeability transition. Biochem J 336(Pt 2):287–290
Xu C, Bailly-Maitre B, Reed JC (2005) Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest 115:2656–2664. doi:10.1172/JCI26373
Yamaguchi H, Wang HG (2004) CHOP is involved in endoplasmic reticulum stress-induced apoptosis by enhancing DR5 expression in human carcinoma cells. J Biol Chem 279:45495–45502. doi:10.1074/jbc.M406933200
Zhang D, Armstrong JS (2007) Bax and the mitochondrial permeability transition cooperate in the release of cytochrome c during endoplasmic reticulum-stress-induced apoptosis. Cell Death Differ 14:703–715. doi:10.1038/sj.cdd.4402072
Zhang D, Lu C, Whiteman M, Chance B, Armstrong JS (2008) The mitochondrial permeability transition regulates cytochrome c release for apoptosis during endoplasmic reticulum stress by remodeling the cristae junction. J Biol Chem 283:3476–3486. doi:10.1074/jbc.M707528200
Zhang PL, Lun M, Teng J, Huang J, Blasick TM, Yin L, Herrera GA, Cheung JY (2004) Preinduced molecular chaperones in the endoplasmic reticulum protect cardiomyocytes from lethal injury. Ann Clin Lab Sci 34:449–457
Zhao ZQ, Vinten-Johansen J (2006) Postconditioning: reduction of reperfusion-induced injury. Cardiovasc Res 70:200–211. pii: S0008-6363(06)00060-5
Zhu T, Au-Yeung KK, Siow YL, Wang G, O K (2002) Cyclosporine A protects against apoptosis in ischaemic/reperfused rat kidneys. Clin Exp Pharmacol Physiol 29:852–854
Acknowledgments
Elise Belaidi was the recipient of a Grant from La Fondation Lefoulon Delalande (France).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
395_2013_363_MOESM1_ESM.pptx
Fig. S1 mRNA content of ER stress markers after inhibition of CypD by NIM811. RT-qPcR on eIF2S1a (a), Grp78 (b), CHOP (c) and spliced-Xbp (Xbps) (d) mRNA in ctrl, NIM and TUDCA + NIM groups. mRNA levels were normalized with murine β-glucuronidase (mGUSB). One-way ANOVA, student Newman–Keuls post hoc tests, ***p < 0.001 vs. ctrl group (PPTX 83 kb)
395_2013_363_MOESM2_ESM.pptx
Fig. S2 mRNA content of ER stress markers after TUDCA treatment alone. RT-qPcR on eIF2S1a (a), Grp78 (b), CHOP (c) and spliced-Xbp (Xbps) (d) mRNA in ctrl and TUDCA groups. mRNA levels were normalized with murine β-glucuronidase (mGUSB). One-way ANOVA, student Newman–Keuls post hoc tests, ***p < 0.001 vs. ctrl group (PPTX 71 kb)
Rights and permissions
About this article
Cite this article
Belaidi, E., Decorps, J., Augeul, L. et al. Endoplasmic reticulum stress contributes to heart protection induced by cyclophilin D inhibition. Basic Res Cardiol 108, 363 (2013). https://doi.org/10.1007/s00395-013-0363-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-013-0363-z