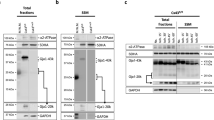
Abstract
Activation of several protein kinases occurs during myocardial ischaemia and during subsequent reperfusion. In contrast to the intensive investigation into the significance of kinase activation in cardioprotection, relatively little is known about the role of the phosphatases in this regard. The aim of this study was to re-evaluate the putative roles of PP1 and PP2A in ischaemia/reperfusion and in triggering ischaemic preconditioning. Isolated perfused working rat hearts were subjected to sustained global (15 or 20 min) or regional ischaemia (35 min), followed by reperfusion. Hearts were preconditioned using global ischaemia (1 × 5 or 3 × 5 min, alternated with 5 min reperfusion). To inhibit both PP1 and PP2A cantharidin (5 μM) was used. To inhibit PP2A only, okadaic acid (7.5 nM) was used. The drugs were administered during the preconditioning protocol, before onset of sustained ischaemia (pretreatment) or during reperfusion. Endpoints were mechanical recovery during reperfusion, infarct size and activation of PKB/Akt, p38 MAPK and ERK p42/p44, as determined by Western blot. Pretreatment of hearts with okadaic acid or cantharidin caused a significant reduction in mechanical recovery after 15 or 20 min global ischaemia. Administration of the drugs during an ischaemic preconditioning protocol abolished functional recovery during reperfusion and significantly increased infarct size. Administration of the drugs during reperfusion had no deleterious effects and increased functional recovery in 3 × PC hearts. To find an explanation for the differential effects of the inhibitors depending on the time of administration, hearts were freeze-clamped at different time points during the perfusion protocol. Administration of cantharidin before 5 min ischaemia activated all kinases. Subsequent reperfusion for 5 min without the drug maintained activation of the kinases until the onset of sustained ischaemia. Cantharidin given during preconditioning was associated with activation of p38MAPK and PKB/Akt during reperfusion after sustained ischaemia. However, administration of the drug during reperfusion only after sustained ischaemia caused activation of both PKB/Akt and ERK p42/p44. Phosphatase inhibition immediately prior to the onset of sustained ischaemia or during preconditioning abolishes protection during reperfusion, while inhibition of these enzymes during reperfusion either had no effect or enhanced the cardioprotective effects of preconditioning. It is proposed that inhibition of phosphatases during reperfusion may prolong the period of RISK activation and hence protect the heart.











Similar content being viewed by others
References
Adams DG, Coffee RL Jr, Zhang H, Pelech S, Strack S, Wadzinski BE (2005) Positive regulation of Raf1-MEK1/2-ERK1/2 signaling by protein serine/threonine phosphatase 2A holoenzymes. J Biol Chem 280:42644–42654
Aikawa R, Komuro I, Yamazaki T, Zou Y, Kudoh S, Tanaka M, Shiojima I, Hiroi Y, Yazaki Y (1997) Oxidative stress activates extracellular signal-regulated kinases through Sre and Ras in cultured cardiac myocytes of neonatal rats. J Clin Invest 100:1813–1821
Anjelkovic M, Jakubowicz T, Cron P, Ming XF, Han JW, Hemmings BA (1996) Activation and phosphorylation of a pleckstrin homology domain containing protein kinase (RAC-PK/PKB) promoted serum and protein phosphatase inhibitors. Proc Nat Acad Sci 193:5699–5709
Avdi NJ, Malcolm KC, Nick JA, Worthen GS (2002) A role for protein phosphatase 2A in p38 mitogen-activated protein kinase-mediated regulation of the c-Jun NH2-terminal kinase pathway in human neutrophils. J Biol chem 277:40687–40696
Armstrong SC, Gao W, Lane JR, Ganote CE (1998) Protein phosphatase inhibitors calyculin A and fostriecin protect rabbit cardiomyocytes in late ischemia. J Mol Cell Cardiol 30:61–73
Barancik M, Htun P, Schaper W (1999) Okadaic acid and anisomycin are protective and stimulate the SAPK/JNK pathway. J Cardiovasc Pharmacol 34:182–190
Bassi R, Heads R, Marber MS, Clark JE (2008) Targeting p38-MAPK in the ischemic heart: kill or cure? Curr Opin Pharmacol 8:141–146
Boudreau RT, Conrad DM, Hoskin DW (2007) Apoptosis induced by protein phosphatase 2A (PP2A) inhibition in T leukemia cells is negatively regulated by PP2A associated p38 mitogen-activated protein kinase. Cell Signal 19:139–151
Bueno DF, Lips DL, Kaiser RA, Wilkens BJ, Dai Y-S, Glascock BJ, Klevitsky R, Hewett TE, Kimball TR, Aronow BJ, Doevendans PA, Molkentin JD (2004) Calcineurin Aβ gene targeting predisposes the myocardium to acute ischemia-induced apoptosis and dysfunction. Circ Res 941:91–99
Cardone MH, Roy N, Stennicke HR, Salvesan GS, Franke TF, Stanbridge E, Frisch S, Reed JC (1998) Regulation of cell death protease caspase-9 by phosphorylation. Science 282:1318–1321
Cai Z, Semenza GL (2005) PTEN activity is modulated during ischemia and reperfusion. Involvement in the induction and decay of preconditioning. Circ Res 97:1351–1359
Cicconi S, Ventura N, Pastore D, Bonini P, Di Navdo P, Lauro R, Marlier LN (2003) Characterization of apoptosis signal transduction pathways in HL-5 cardiomyocytes exposed to ischemia/reperfusion oxidative stress. J Cell Physiol 195:27–37
Cohen P, Cohen PT (1989) Protein phosphatases come of age. J Biol Chem 264:21435–21438
Crompton M, Virji S, Ward JM (1998) Cyclophilin-D binds strongly to complexes of the voltage-dependent anion channel and the adenine nucleotide translocase to form the permeability transition pore. J Eur Biochem 258:729–735
Cross TG, Scheel-Toellner D, Henriquez NV, Deacon E, Salmon M, Lord JM (2000) Serine/threonine protein kinases and apoptosis. Exp Cell Res 256:34–41
Das M, Das DK (2008) Molecular mechanism of preconditioning. IUBMB Life 60:199–203
De Windt LJ, Lim HW, Taigen T, Wencker D, Condorelli G, Dorn GW, Kitsis RN, Molkentin JD (2000) Calcineurin-mediated hypertrophy protects cardiomyocytes from apoptosis in vitro and in vivo; an apoptosis-independent model of dilated heart failure. Circ Res 86:255–263
Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM (1999) Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399:601–605
Downward J (1999) How BAD phosphorylation is good for survival. Nat Cell Biol 1:E33–E35
Fan W-J, Genade S, Genis A, Huisamen B, Lochner A (2009) Dexamethasone-induced cardioprotection: a role for the phosphatase MKP-1? Life Sci 84:838–846
Faris B, Peyner J, Wassef M, Bel A, Mouas C, Duriez M, Menasche P (1997) Failure of preconditioning to improve postcardioplegic stunning of minimally infarcted hearts. Ann Thorac Surg 64:1735–1741
Fenton RA, Dickson EW, Dobson JG (2005) Inhibition of phosphatase activity enhances preconditioning and limits cell death in the ischemic/reperfused aged rat heart. Life Sci 77:3375–3388
Fryer RM, Pratt PF, Hsu AK, Gross GJ (2001) Differential activation of extracellular signal-regulated kinase isoforms in preconditioning and opioid-induced cardioprotection. J Pharmacol Exp Ther 296:642–649
Gombosova I, Boknik P, Kirchhefer U, Knapp U, Luss H, Muller FU, Vahlensieck U, Schmitz W, Bodor GS, Neumann J (1998) Postnatal changes in contractile time parameters, calcium regulatory proteins and phosphatases. Am J Physiol 274:2123–2132
Hardt SE, Sadoshima J (2002) Glycogen synthase kinase-3β: a novel regulator of cardiac hypertrophy and development. Circ Res 90:1055–1063
Hausenloy DJ, Tsang A, Mocanu MM, Yellon DM (2005) Ischemic preconditioning protects by activating prosurvival kinases at reperfusion. Am J Physiol Heart Circ Physiol 288:H971–H976
Hausenloy DJ, Tsang A, Yellon DM (2005) The reperfusion injury salvage kinase pathway: a common target for both ischaemic preconditioning and postconditioning. Trends Cardiovasc Med 15:69–75
Hausenloy DJ, Yellon DM, Mani-Babu S, Duchen MR (2004) Preconditioning protects by inhibiting the mitochondrial permeability transition. Am J Physiol Heart Circ Physiol 287:841–849
Heusch G (2009) No RISK, no… cardioprotection? A clinical perspective. Cardiovasc Res 84:173–175
Heusch G, Boengler R, Schulz R (2008) Cardioprotection. Nitric oxide, protein kinase and mitochondria. Circulation 118:1915–1919
Herzig S, Neumann J (2000) Effects of serine/threonine protein phosphatases on ion channels in excitable membranes. Physiol Rev 80:173–210
Huang B, Wang S, Qin D, Bontjdir M, El-Sherif N (1999) Diminished basal phosphorylation level of phospholamban in the postinfarction remodeled rat ventricle: role of beta-adrenergic pathway, Gi protein, phosphodiesterase and phosphatases. Circ Res 85:948–953
Ingebritsen TS, Stewart AA, Cohen P (1983) The protein phosphatases involved in cellular regulation. 6. Measurement of type-1 and type-2 protein phosphatases in extracts of mammalian tissues; an assessment of their physiological roles. J Eur Biochem 132:297–307
Ishihara H, Martin BL, Brautigan DL, Karaki H, Ozaki H, Kato Y, Fusentani N, Watabe S, Hashimoto K, Nemura D, Hartshorne DJ (1989) CalyculinA and okadaic acid: inhibitors of protein phosphatase activity. Biochem Biophys Res Commun 159:871–877
Iwakara A, Fujita M, Hasegawa K, Toyokuni S, Sawamura T, Nohara R, Sasayama S, Komeda M (2001) Pericardial fluid from patients with ischemic heart disease induces myocardial cell apoptosis via an oxidant stress-sensitive p38 mitogen-activated protein kinase pathway. J Mol Cell Cardiol 33:419–430
Jenkins DP, Bugsley WB, Yellon DM (1995) Ischaemic preconditioning in a model of global ischaemia: infarct size limitation but no reduction in stunning. J Mol Cell Cardiol 27:1623–1632
Juntilla MR, Li S-P, Westermarck J (2008) Phosphatase-mediated crosstalk between MAPK signaling pathways in the regulation of cell survival. FASEB J 22:954–965
Kannengieser GJ, Opie LH, Van der Werff TJ (1979) Impaired cardiac work and oxygen uptake after reperfusion of regionally ischaemic myocardium. J Mol Cell Cardiol 11:197–207
Ladilov Y, Maxeiner H, Wolf C, Scäfer C, Meuter K, Piper HM (2002) Role of protein phosphatases in hypoxic preconditioning. Am J Physiol Heart Circ Physiol 283:H1092–H1098
Latronico M, Costinean S, Latitrano ML, Peschle C, Condorelli G (2004) Regulation of cell size and contractile function by Akt in cardiomyocytes. Ann NY Acad Sci 1015:250–260
Liem DA, Gho CC, Gho BC, Kazim S, Manintveld OC, Verdouw PD, Dunker DJ (2004) The tyrosine phosphatase inhibitor B15 (maltolato) oxovanadium attenuates myocardial reperfusion injury by opening ATP-sensitive potassium channels. J Pharm Exp Ther 309:1256–1262
Linck B, Boknik P, Knapp J, Muller U, Neumann J, Schmitz W, Vahlensieck U (1996) Effects of cantharidin on force of contraction and phosphatase activity in nonfailing and failing human hearts. Br J Pharmacol 119:545–550
Liu Q, Hofmann PA (2003) Modulation of protein phosphatase 2a by adenosine A1 receptors in cardiomyocytes: role for p38MAPK. J Am Physiol Heart Circ Physiol 285:97–103
Lochner A, Genade S, Moolman JA (2003) Ischemic preconditioning: infarct size is a more reliable endpoint than functional recovery. Basic Res Cardiol 98:337–346
Lu C, Kumar R, Akita T, Joyner RW (1994) Developmental changes in the actions of phosphatase inhibitors on calcium current of rabbit heart cells. Pflugers Arch 427:389–398
Ma XL, Kumar S, Gao F, Londen CS, Lopez BL, Christopher TA, Wang C, Lee JC, Fenarstein GZ, Yue TL (1999) Inhibition of p38 mitogen-activated protein kinase decreases cardiomyocyte apoptosis and improves cardiac function after myocardial ischemia and reperfusion. Circulation 99:1685–1691
MacKay K, Mochley-Rosen D (2000) Involvement of a p38 mitogen-activated protein kinase phosphatase in protecting neonatal rat cardiac myocytes from ischemia. J Mol Cell Cardiol 32:1585–1588
Marais E, Genade S, Huisamen B, Strijdom JG, Moolman JA, Lochner A (2001) Activation of p38MAPK induced by a multi-cycle ischaemic preconditioning protocol is associated by attenuated p38MAPK activity during sustained ischaemia and reperfusion. J Mol Cell Cardiol 33:769–778
Marais E, Genade S, Salie R, Huisamen B, Maritz S, Moolman JA, Lochner A (2005) The temporal relationship between p38MAPK and HSP27 activation in ischaemic and pharmacological preconditioning. Basic Res Cardiol 100:35–47
Gehringer MM(2004) Microcystin-LR and okadaic acid induced cellular effects: a dualistic response. Febs Lett 557:1–8
Moolman JA, Hartley S, Van Wyk J, Marais E, Lochner A (2006) Inhibition of myocardial apoptosis by ischaemic and beta-adrenergic preconditioning is dependent on p38MAPK. Cardiovasc Drugs Ther 20:13–25
Morana S, Wolf CM, Li J, Reynolds JE, Brown MK, Eastman A (1996) The involvement of protein phosphatases in the activation of ICE/CED-3 protease, intracellular acidification, DNA digestion and apoptosis. J Biol Chem 271:18263–18271
Neumann J, Herzig S, Boknik P, Apel M, Kaspareit G, Schmitz W, Scholz H, Tepel M, Zimmerman N (1995) On the contractile, biochemical and electrophysiological effects of cantharidin, a phosphatase inhibitor. J Pharmacol Exp Ther 274:530–539
Prickett TD, Brautigan DL (2007) Cytokine activation of p38 mitogen-activated protein kinase and apoptosis is opposed by alpha-4 targeting of protein phosphatase 2A for site-specific dephosphorylation of MEK3. Mol Cell Biol 27:4217–4227
Przyklenk K, Kloner RA (1998) Ischemic preconditioning: exploring the paradox. Prog Cardiovasc Dis 40:517–547
Ruan H, Li J, Ren S, Gao J, Li G, Kim R, Wu H, Wang Y (2009) Inducible and cardiac specific PTEN inactivation protects ischemia/reperfusion injury. J Mol Cell Cardiol 46:193–200
Sanada S, Kitakaze M, Papst PJ, Hatanaka K, Asanuma H, Aki T, Shinozaki Y, Ogita H, Node K, Takashima S, Asakura M, Yamoda J, Fukushima T, Ogai A, Kuzuya T, Mori H, Terada N, Yoshida K, Hori M (2001) Role of phasic dynamism of p38 mitogen activated protein kinase activation in ischemic preconditioning of the canine heart. Circ Res 88:175–180
Sanna B, Bueno DF, Dai YS, Wilkens BJ, Molkentin JD (2005) Direct and indirect interactions between calcineurin-NFAT and MEK1-extracellular signal-regulated kinase 1/2 signaling pathways regulate cardiac gene expression and cellular growth. Mol Cell Biol 25:865–878
Schneider S, Chen W, Hon J, Steenbergen C, Murphy E (2001) Inhibition of p38MAPK α/β reduces ischemic injury and does not block protective effects of preconditioning. Am J Physiol 280:H499–H508
Siddall HK, Warrell CE, Yellon DM, Mocanu MM (2008) Ischemia–reperfusion injury and cardioprotection: investigating PTEN, the phosphatase that negatively regulates PI3K, using a congenital model of PTEN haploinsufficiency. Basic Res Cardiol 103:560–568
Steenbergen C (2002) The role of p38 mitogen-activated protein kinase in myocardial ischaemia/reperfusion injury: relationship to ischemic preconditioning. Basic Res Cardiol 97:276–285
Stenslokken KO, Rutkovskiy A, Hafstad AD, Larsen TS, Vaage J (2009) Inadvertent phosphorylation of survival kinases in isolated perfused hearts: a word of caution. Basic Res Cardiol 104:412–423
Sontag E, Fedorov S, Kamibayashi C, Robbins D, Cobb M, Mumby M (1993) The interaction of SV40 small tumor antigen with protein phosphatase A2 stimulates the map kinase pathway and induces cell proliferation. Cell 75:887–897
Totzeck A, Boengler K, Van de Sand A, Konietzka I, Gres P, Garcia-Dorado D, Heusch G, Schulz R (2008) No impact of phosphatases on connexin 43 phosphorylation in ischemic preconditioning. Am J Physiol Heart Circ Physiol 295:H2106–H2112
Ugi S, Imamura T, Maegawa H, Yoshizaki T, Shi K, Obata T, Ebina Y, Kashiwagi A, Olefsky JM (2004) Protein phosphatase 2A negatively regulates insulin’s metabolic signaling pathway by inhibiting Akt (protein kinase B) activity in 3T3-L1 adipocytes. Mol Cell Biol 24:8778–8789
Vahlhaus C, Schulz R, Post H, Rose J, Heusch G (1998) Prevention of ischemic preconditioning only by combined inhibition of protein kinase C and protein tyrosine kinase in pigs. J Mol Cell Cardiol 30:197–209
Weinbrenner C, Baines CP, Liu GS, Armstrong SC, Ganote CE, Walsh AH, Honkanen RE, Cohen MV, Downey JM (1999) Fostriecin, an inhibitor of protein phosphatase 2A, limits myocardial infarct size even when administered after onset of ischemia. Circulation 98:899–905
Westermarck J, Li SP, Kallunki T, Han J, Kahari VM (2001) p38-mitogen activated protein kinase-dependent activation of protein phosphatases 1 and 2A inhibits MEK1 and MEK2 activity and collagenase 1 (MMP-1) gene expression. Mol Cell Biol 21:2373–2383
Wolf CM, Eastman A (1999) The temporal relationship between protein phosphatase, mitochondrial cytochrome C release and caspase activation in apoptosis. Exp Cell Res 247:505–513
Yue TL, Wang C, Gu JL, Ma XL, Kumar S, Lee JC, Feuerstein GZ, Thomas H, Maleeff B, Ohlstein EH (2000) Inhibition of extracellular signal-regulated kinase enhances ischemia/reoxygenation-induced apoptosis in cultured cardiac myocytes and exaggerates reperfusion injury in isolated perfused rat heart. Circ Res 86:692–699
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fan, W.J., van Vuuren, D., Genade, S. et al. Kinases and phosphatases in ischaemic preconditioning: a re-evaluation. Basic Res Cardiol 105, 495–511 (2010). https://doi.org/10.1007/s00395-010-0086-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00395-010-0086-3