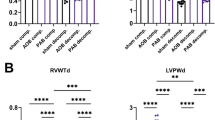
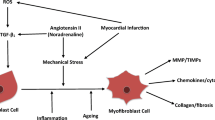
Abstract
Gap junctions are intercellular channels that connect the cytoplasm of adjacent cell. Gap junctional intercellular communication has long been postulated to contribute to the maintenance of tissue homeostasis. Recent studies, however, have demonstrated that connexins, gap junction proteins, are involved in the regulation of a variety of cellular functions other than intercellular communication. Although, in neonatal rat ventricular myocytes, connexin-40, -43, and -45 are all expressed, connexin43 (Cx43) is the primary subtype. In this study, we examined whether and if so how the knockdown of a gap junction protein Cx43 with siRNA produced changes in the proliferative activity of neonatal cardiomyocytes. Cx43-knockdown resulted in a significant increase in the proliferation of cardiomyocytes. To clarify the mechanisms behind this increase, we investigated whether the activity of mitogen-activated protein kinases (MAPKs) changed on knockdown of Cx43. The knockdown decreased the expression of phosphorylated p38 (p-p38) MAPK. In addition, treatment of cardiomyocytes with a p38 MAPK inhibitor significantly increased the proliferative activity. Cultures were then co-treated with an inhibitor of p38 MAPK and fibroblast growth factor-1 (FGF1), since Cx43-knockdown significantly increased cytosolic FGF1 expression as well. The co-treatment enhanced the proliferation of cardiomyocytes compared with the treatment with the p38 MAPK inhibitor alone. Taken together, the present study demonstrated that Cx43-knockdown produced a significant increase in the proliferation of neonatal cardiomyocytes.







Similar content being viewed by others
References
Alexander DB, Goldberg GS (2003) Transfer of biologically important molecules between cells through gap junction channels. Curr Med Chem 10:2045–2058
Ambrosino C, Nebreda AR (2001) Cell cycle regulation by p38 MAP kinases. Biol Cell 93:47–51
Bers DB, Jongsma HJ (1996) Connexins in mammalian heart function. Bioessays 18:719–730
Boengler K, Dodoni G, Rodriguez-Sinovas A, Cabestrero A, Ruiz-Meana M, Gres P, Konietzka I, Lopez-Iglesias C, Garcia-Dorado D, Lisa FD, Heusch G, Schulz R (2005) Connexin 43 in cardiomyocyte mitochondria and its increase by ischemic preconditioning. Cardiovasc Res 67:234–244
Bulavin DV, Amundson SA, Fornace AJ (2002) p38 and Chk1 kinases: different conductors for the G2/M checkpoint symphony. Curr Opin Genet Dev 12:92–97
Dai P, Nakagami T, Tanaka H, Hitomi T, Takamatsu T (2007) Cx43 mediates TGF-β signaling through competitive Smads binding to microtubules. Mol Biol Cell 18:2264–2273
Dang X, Doble BW, Kardami E (2003) The carboxy-tail of connexin-43 localizes to the nucleus and inhibits cell growth. Mol Cell Biochem 242:35–38
Dang X, Jeyaraman M, Kardami E (2006) Regulation of connexin-43-mediated growth inhibition by a phosphorylatable amino-acid is independent of gap junction-forming ability. Mol Cell Biochem 289:201–207
Dhein S, Pejman P, Krüsemann K (2000) Effects of IK.ATP blockers glibenclamide and HMR 1883 on cardiac electrophysiology during ischemia and reperfusion. Eur J Pharmacol 398:273–284
Doble BW, Dang X, Ping P, Fandrich RR, Nickel BE, Jin Y, Cattini PA, Kardami E (2004) Phosphorylation of serine 262 in the gap junction protein connexin-43 regulates DNA synthesis in cell-cell contact forming cardiomyocytes. J Cell Sci 117:507–514
Dost T, Cohen MV, Downey JM (2008) Redox signaling triggers protection during the reperfusion rather than the ischemic phase of preconditioning. Basic Res Cardiol 103:378–384
Engel FB, Schebesta M, Duong MT, Lu G, Ren S, Madwed JB, Jiang H, Wang Y, Keating MT (2005) p38 MAP kinase inhibition enables proliferation of adult mammalian cardiomyocytes. Genes Dev 19:1175–1187
Engelmann GL, Dionne CA, Jaye MC (1993) Acidic fibroblast growth factor and heart development: role in myocyte proliferation and capillary angiogenesis. Circ Res 72:7–19
Frantz S, Hu K, Adamek A, Wolf J, Sallam A, Maier SKG, Lonning S, Ling H, Ertl G, Bauersachs J (2008) Transforming growth factor beta inhibition increases mortality and left ventricular dilatation after myocardial infarction. Basic Res Cardiol 103:485–492
Fromaget G, Aoumari AE, Dupont E, Briand JP, Gros D (1990) Changes in the expression of connexin 43, a cardiac gap junctional protein, during mouse heart development. J Mol Cell Cardiol 22:1245–1258
Garcia-Dorado D, Ruiz-Meana M (2000) Propagation of cell death during myocardial reperfusion. News Physiol Sci 15:326–330
Giepmans BN (2004) Gap junctions and connexin-interacting proteins. Cardiovasc Res 62:233–245
Giepmans BN, Verlaan I, Hengeveld T, Janssen H, Calafat J, Falk MM, Moolenaar WH (2001) Gap junction protein connexin-43 interacts directly with microtubules. Curr Biol 11:1364–1368
Heinzel FR, Luo Y, Dodoni G, Boengler K, Petrat F, Lisa FD, de Groot H, Schulz R, Heusch G (2006) Formation of reactive oxygen species at increased contraction frequency in rat cardiomyocytes. Cardiovasc Res 71:374–382
Hirschi KK, Burt JM, Hirschi KD, Dai C (2003) Gap junction communication mediates transforming growth factor-β activation and endothelial-induced mural cell differentiation. Circ Res 93:429–437
Iacobas DA, Scemes E, Spray DC (2004) Gene expression alterations in connexin null mice extend beyond the gap junction. Neurochem Int 45:243–250
Kardami E, Dang X, Iacobas DA, Nickel BE, Jeyaraman M, Srisakuldee W, Makazan J, Tanguy S, Spray DC (2007) The role of connexins in controlling cell growth and gene expression. Prog Biophys Mol Biol 94:245–264
Kawahara K, Abe R, Yamauchi Y, Kohashi M (2002) Fluctuations of contraction rhythm during simulated ischemia/reperfusion in cultured cardiac myocytes from neonatal rats. Biol Rhythm Res 33:339–350
Kawahara K, Hachiro T, Yokokawa T, Nakajima T, Yamauchi Y, Nakayama Y (2006) Ischemia/reperfusion-induced death of cardiac myocytes: possible involvement of nitric oxide in the coordination of ATP supply and demand during ischemia. J Mol Cell Cardiol 40:35–46
Kohashi M, Kawahara K, Yamauchi Y (2003) Carbachol-induced suppression of contraction rhythm in spontaneously beating cultured cardiac myocytes from neonatal rats. Biol Rhythm Res 34:367–381
Kramer RM, Roberts EF, Um SL, Börsch-Haubold AG, Watson SP, Fisher MJ, Jakubowski JA (1996) p38 mitogen-activated protein kinase phosphorylates cytosolic phospholipase A2 (cPLA2) in thrombin-stimulated platelets. J Biol Chem 271:27723–27729
Kulisz A, Chen N, Chandel NS, Shao Z, Schumacker PT (2002) Mitochondrial ROS initiate phosphorylation of p38 Map kinase during hypoxia in cardiomyocytes. Am J Physiol 282:L1324–1329
Kumar NM, Gilula NB (1996) The gap junction communication channel. Cell 84:381–388
Li Z, Jiang Y, Ulevitch RJ, Han J (1996) The primary structure of p38γ: a new member of p38 group of MAP kinases. Biochem Biophys Res Commun 228:334–340
Liang Q, Molkentin JD (2003) Redefining the roles of p38 and JNK signaling in cardiac hypertrophy: dichotomy between cultured myocytes and animal models. J Mol Cell Cardiol 35:1385–1394
Lin Q, Schwarz J, Bucana C, Olson EN (1997) Control of mouse cardiac morphogenesis and myogenesis by transcription factor MEF2C. Science 276:1404–1407
Matsuoka-Ida M, Takimoto Y, Aoyama T, Akao M, Takeda T, Kita T (2006) Activation of TGF-β1-TAK1–p38 MAPK pathway in spared cardiomyocytes is involved in left ventricular remodeling after myocardial infarction in rats. Am J Physiol 290:H709–715
Matsuyama D, Kawahara K (2008) Maintenance and characterization of spontaneous contraction rhythm in cultured cardiac myocytes fused with cardiac fibroblasts. Biosystems 92:226–232
McGill CJ, Brooks G (1995) Cell cycle control mechanisms and their role in cardiac growth. Cardiovasc Res 30:557–569
Omori Y, Yamasaki H (1999) Gap junction proteins connexin32 and connexin43 partially acquire growth-suppressive function in HeLa cells by deletion of their C-terminal tails. Carcinogenesis 20:1913–1918
Parker TG, Packer SE, Schneider MD (1990) Peptide growth factors can provoke “fetal” contractile protein gene expression in rat cardiac myocytes. J Clin Invest 85:507–514
Pines J, Hunter T (1991) Human cyclins A and B1 are differentially located in the cell and undergo cell cycle-dependent nuclear transport. J Cell Biol 115:1–17
Rama A, Matsushita T, Charolidi N, Rothery S, Dupont E, Severs NJ (2006) Up-regulation of connexin43 correlates with increased synthetic activity and enhanced contractile differentiation in TGF-β-treated human aortic smooth muscle cells. Eur J Cell Biol 85:375–386
Ruiz-Meana M, Rodriguez-Sinovas A, Cabestrero A, Boengler K, Heusch G, Garcia-Dorado D (2008) Mitochondrial connexin43 as a new player in the pathophysiology of myocardial ischemia-reperfusion injury. Cardiovasc Res 77:325–333
Ruusalepp A, Czibik G, Flatebϕ T, Vaage J, Valen G (2007) Myocardial protection evoked by hyperoxic exposure involves signaling through nitric oxide and mitogen activated protein kinases. Basic Res Cardiol 102:318–326
Salameh A, Mühlberg K, Schneider P, Dhein S, Pfeiffer D (2005) Differential regulation of gap junction proteins connexin 40 and connexin 43 in cardiomyocytes. Intern J Pharmacol 1:44–54
Salameh A, Krautblatter S, Baeβler S, Karl S, Gomez DR, Dhein S, Pfeiffer D (2008) Signal transduction and transcriptional control of cardiac connexin43 up-regulation after α1-adrenoceptor stimulation. J Pharmacol Exp Ther 326:315–322
Schulz R, Gres P, Skyschally A, Duschin A, Belosjorow S, Konietzka I, Heusch G (2003) Ischemic preconditioning preserves connexin 43 phosphorylation during sustained ischemia in pig hearts in vivo. FASEB J 17:1355–1357
Shi Y, Gaestel M (2002) In the cellular garden of forking paths: how p38 MAPKs signal for downstream assistance. Biol Chem 383:1519–1536
Sucharov CC, Mariner PD, Nunley KR, Long C, Leinwand L, Bristow MR (2006) A β1-adrenergic receptor CAM kinase II dependent pathway mediates cardiac myocyte fetal gene induction. Am J Physiol 291:H1299–1308
Tacheau C, Fontaine J, Loy J, Mauviel A, Verrecchia F (2008) TGF-β induces connexin43 gene expression in normal murine mammary gland epithelial cells via activation of p38 and PI3 K/AKT signaling pathways. J Cell Physiol 217:759–768
Vink MJ, Suadicani SO, Vieira DM, Yrban-Maldonado M, Gao Y, Fishman GI, Spray DC (2004) Alterations of intercellular communication in neonatal cardiac myocytes from connexin-43 null mice. Cardiovasc Res 62:397–406
Zhu S, Goldschmidt-Clermont PJ, Dong C (2004) Transforming growth factor-β-induced inhibition of myogenesis is mediated through Smad pathway and is modulated by microtubule dynamic stability. Circ Res 19:617–625
Acknowledgments
This work was supported in part by a grant-in-aid for scientific research from the Ministry of Education, Science, and Culture of Japan (16300145, 19300153) to KK.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Matsuyama, D., Kawahara, K. Proliferation of neonatal cardiomyocytes by connexin43 knockdown via synergistic inactivation of p38 MAPK and increased expression of FGF1. Basic Res Cardiol 104, 631–642 (2009). https://doi.org/10.1007/s00395-009-0029-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00395-009-0029-z