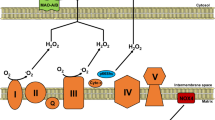
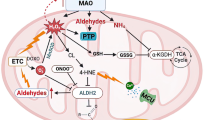
Abstract
Although mitochondria are considered the most relevant site for the formation of reactive oxygen species (ROS) in cardiac myocytes, a major and unsolved issue is where ROS are generated in mitochondria. Respiratory chain is generally indicated as a main site for ROS formation. However, other mitochondrial components are likely to contribute to ROS generation. Recent reports highlight the relevance of monoamine oxidases (MAO) and p66Shc. The importance of these systems in the irreversibility of ischemic heart injury will be discussed along with the cardioprotective effects elicited by both MAO inhibition and p66Shc knockout. Finally, recent evidence will be reviewed that highlight the relevance of mitochondrial ROS formation also in myocardial failure and atherosclerosis.

Similar content being viewed by others
References
Andoh T, Lee SY, Chiueh CC (2000) Preconditioning regulation of bcl-2 and p66Shc by human NOS1 enhances tolerance to oxidative stress. FASEB J 14:2144–2146
Asimakis GK, Lick S, Patterson C (2002) Postischemic recovery of contractile function is impaired in SOD2(+/–) but not SOD1(+/–) mouse hearts. Circulation 105:981–986
Aulak KS, Miyagi M, Yan L, West KA, Massillon D, Crabb JW, Stuehr DJ (2001) Proteomic method identifies proteins nitrated in vivo during inflammatory challenge. Proc Natl Acad Sci USA 98:12056–12061
Baines CP (2009) The mitochondrial permeability transition pore and ischemia-reperfusion injury. Basic Res Cardiol (in press)
Balaban RS, Nemoto S, Finkel T (2005) Mitochondria, oxidants, and aging. Cell 120:483–495
Bauersachs J, Widder JD (2008) Endothelial dysfunction in heart failure. Pharmacol Rep 60:119–126
Beckman JS, Koppenol WH (1996) Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol 271:C1424–C1437
Beltran B, Mathur A, Duchen MR, Erusalimsky JD, Moncada S (2000) The effect of nitric oxide on cell respiration: a key to understanding its role in cell survival or death. Proc Natl Acad Sci USA 97:14602–14607
Bernardi P, Petronilli V, Di Lisa F, Forte M (2001) A mitochondrial perspective on cell death. Trends Biochem Sci 26:112–117
Bernardi P, Krauskopf A, Basso E, Petronilli V, Blalchy-Dyson E, Di Lisa F, Forte MA (2006) The mitochondrial permeability transition from in vitro artifact to disease target. FEBS J 273:2077–2099
Berndt C, Lillig CH, Holmgren A (2007) Thiol-based mechanisms of the thioredoxin and glutaredoxin systems: implications for diseases in the cardiovascular system. Am J Physiol Heart Circ Physiol 292:H1227–H1236
Berniakovich I, Trinei M, Stendardo M, Migliaccio E, Minucci S, Bernardi P, Pelicci PG, Giorgio M (2008) p66Shc-Generated oxidative signal promotes fat accumulation. J Biol Chem 283:34283–34293
Bianchi P, Kunduzova O, Masini E, Cambon C, Bani D, Raimondi L, Seguelas MH, Nistri S, Colucci W, Leducq N, Parini A (2005) Oxidative stress by monoamine oxidase mediates receptor-independent cardiomyocyte apoptosis by serotonin and postischemic myocardial injury. Circulation 112:3297–3305
Bianchi P, Pimentel DR, Murphy MP, Colucci WS, Parini A (2005) A new hypertrophic mechanism of serotonin in cardiac myocytes: receptor-independent ROS generation. FASEB J 19:641–643
Bolli R, Marban E (1999) Molecular and cellular mechanisms of myocardial stunning. Physiol Rev 79:609–634
Booz GW (2005) Growing old, angiotensin II, cardiac hypertrophy, and death: making the connection with p66Shc. Hypertension 46:259–260
Brennan JP, Bardswell SC, Burgoyne JR, Fuller W, Schroder E, Wait R, Begum S, Kentish JC, Eaton P (2006) Oxidant-induced activation of type I protein kinase A is mediated by RI subunit interprotein disulfide bond formation. J Biol Chem 281:21827–21836
Brown GC, Cooper CE (1994) Nanomolar concentrations of nitric oxide reversibly inhibit synaptosomal respiration by competing with oxygen at cytochrome oxidase. FEBS Lett 356:295–298
Brunner HG, Nelen M, Breakefield XO, Ropers HH, van Oost BA (1993) Abnormal behavior associated with a point mutation in the structural gene for monoamine oxidase A. Science 262:578–580
Burgoyne JR, Madhani M, Cuello F, Charles RL, Brennan JP, Schroder E, Browning DD, Eaton P (2007) Cysteine redox sensor in PKGIa enables oxidant-induced activation. Science 317:1393–1397
Cadenas E, Davies KJ (2000) Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med 29:222–230
Canton M, Neverova I, Menabò R, Van Eyk JE, Di Lisa F (2004) Evidence of myofibrillar protein oxidation induced by postischemic reperfusion in isolated rat hearts. Am J Physiol Heart Circ Physiol 286:H870–H877
Cases O, Seif I, Grimsby J, Gaspar P, Chen K, Pournin S, Muller U, Aguet M, Babinet C, Shih JC (1995) Aggressive behavior and altered amounts of brain serotonin and norepinephrine in mice lacking MAO-A. Science 268:1763–1766
Cesselli D, Jakoniuk I, Barlucchi L, Beltrami AP, Hintze TH, Nadal-Ginard B, Kajstura J, Leri A, Anversa P (2001) Oxidative stress-mediated cardiac cell death is a major determinant of ventricular dysfunction and failure in dog dilated cardiomyopathy. Circ Res 89:279–286
Chen Z, Siu B, Ho YS, Vincent R, Chua CC, Hamdy RC, Chua BH (1998) Overexpression of MnSOD protects against myocardial ischemia/reperfusion injury in transgenic mice. J Mol Cell Cardiol 30:2281–2289
Chen CH, Budas GR, Churchill EN, Disatnik MH, Hurley TD, Mochly-Rosen D (2008) Activation of aldehyde dehydrogenase-2 reduces ischemic damage to the heart. Science 321:1493–1495
Clarke SJ, Khaliulin I, Das M, Parker JE, Heesom KJ, Halestrap AP (2008) Inhibition of mitochondrial permeability transition pore opening by ischemic preconditioning is probably mediated by reduction of oxidative stress rather than mitochondrial protein phosphorylation. Circ Res 102:1082–1090
Cleeter MW, Cooper JM, rley-Usmar VM, Moncada S, Schapira AH (1994) Reversible inhibition of cytochrome c oxidase, the terminal enzyme of the mitochondrial respiratory chain, by nitric oxide. Implications for neurodegenerative diseases. FEBS Lett 345:50–54
Coatrieux C, Sanson M, Negre-Salvayre A, Parini A, Hannun Y, Itohara S, Salvayre R, Auge N (2007) MAO-A-induced mitogenic signaling is mediated by reactive oxygen species, MMP-2, and the sphingolipid pathway. Free Radic Biol Med 43:80–89
Cosentino F, Francia P, Camici GG, Pelicci PG, Luscher TF, Volpe M (2008) Final common molecular pathways of aging and cardiovascular disease: role of the p66Shc protein. Arterioscler Thromb Vasc Biol 28:622–628
Costa AD, Quinlan CL, Andrukhiv A, West IC, Jaburek M, Garlid KD (2006) The direct physiological effects of mitoK(ATP) opening on heart mitochondria. Am J Physiol Heart Circ Physiol 290:H406–H415
Costantini P, Chernyak BV, Petronilli V, Bernardi P (1995) Selective inhibition of the mitochondrial permeability transition pore at the oxidation-reduction sensitive dithiol by monobromobimane. FEBS Lett 362:239–242
Costantini P, Chernyak BV, Petronilli V, Bernardi P (1996) Modulation of the mitochondrial permeability transition pore by pyridine nucleotides and dithiol oxidation at two separate sites. J Biol Chem 271:6746–6751
Daiber A, Wenzel P, Oelze M, Schuhmacher S, Jansen T, Munzel T (2008) Mitochondrial aldehyde dehydrogenase (ALDH-2)-Maker of and marker for nitrate tolerance in response to nitroglycerin treatment. Chem Biol Interact (in press)
Davidson SM, Duchen MR (2007) Endothelial mitochondria: contributing to vascular function and disease. Circ Res 100:1128–1141
Di Lisa F, Bernardi P (2005) Mitochondrial function and myocardial aging. A critical analysis of the role of permeability transition. Cardiovasc Res 66:222–232
Di Lisa F, Menabò R, Canton M, Petronilli V (1998) The role of mitochondria in the salvage and the injury of the ischemic myocardium. Biochim Biophys Acta 1366:69–78
Di Lisa F, Canton M, Menabò R, Dodoni G, Bernardi P (2003) Mitochondria and reperfusion injury. The role of permeability transition. Basic Res Cardiol 98:235–241
Droge W (2002) Free radicals in the physiological control of cell function. Physiol Rev 82:47–95
Dworakowski R, Alom-Ruiz SP, Shah AM (2008) NADPH oxidase-derived reactive oxygen species in the regulation of endothelial phenotype. Pharmacol Rep 60:21–28
Edmondson DE, Binda C, Mattevi A (2004) The FAD binding sites of human monoamine oxidases A and B. Neurotoxicology 25:63–72
Edmondson DE, Mattevi A, Binda C, Li M, Hubalek F (2004) Structure and mechanism of monoamine oxidase. Curr Med Chem 11:1983–1993
Erickson JR, Joiner ML, Guan X, Kutschke W, Yang J, Oddis CV, Bartlett RK, Lowe JS, O’Donnell SE, ykin-Burns N, Zimmerman MC, Zimmerman K, Ham AJ, Weiss RM, Spitz DR, Shea MA, Colbran RJ, Mohler PJ, Anderson ME (2008) A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell 133:462–474
Ferdinandy P, Schulz R, Baxter GF (2007) Interaction of cardiovascular risk factors with myocardial ischemia/reperfusion injury, preconditioning, and postconditioning. Pharmacol Rev 59:418–458
Finetti F, Pellegrini M, Ulivieri C, Savino MT, Paccagnini E, Ginanneschi C, Lanfrancone L, Pelicci PG, Baldari CT (2008) The proapoptotic and antimitogenic protein p66Shc acts as a negative regulator of lymphocyte activation and autoimmunity. Blood 111:5017–5027
Finkel T (2000) Redox-dependent signal transduction. FEBS Lett 476:52–54
Fiorina P, Corradi D, Pinelli S, Maestri R, Lagrasta C, Buscaglia M, Davalli A, Folli F, Astorri E (2004) Apoptotic/mytogenic pathways during human heart development. Int J Cardiol 96:409–417
Flohe L, Ursini F (2008) Peroxidase: a term of many meanings. Antioxid Redox Signal 10:1485–1490
Fridovich I (1995) Superoxide radical and superoxide dismutases. Annu Rev Biochem 64:97–112
Giordano FJ (2005) Oxygen, oxidative stress, hypoxia and heart failure. J Clin Invest 115:500–508
Giorgio M, Migliaccio E, Orsini F, Paolucci D, Moroni M, Contursi C, Pelliccia G, Luzi L, Minucci S, Marcaccio M, Pinton P, Rizzuto R, Bernardi P, Paolucci F, Pelicci PG (2005) Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell 122:221–233
Giorgio M, Trinei M, Migliaccio E, Pelicci PG (2007) Hydrogen peroxide: a metabolic by-product or a common mediator of ageing signals? Nat Rev Mol Cell Biol 8:722–728
Glab M, Lojek A, Wrzosek A, Dolowy K, Szewczyk A (2006) Endothelial mitochondria as a possible target for potassium channel modulators. Pharmacol Rep 58(Suppl):89–95
Gordon LI, Burke MA, Singh AT, Prachand S, Lieberman ED, Sun L, Naik TJ, Naga Prasad SV, Ardehali H (2008) Blockade of the erbB2 receptor induces cardiomyocyte death through mitochondrial- and reactive oxygen species-dependent pathways. J Biol Chem (in press)
Graiani G, Lagrasta C, Migliaccio E, Spillmann F, Meloni M, Madeddu P, Quaini F, Padura IM, Lanfrancone L, Pelicci P, Emanueli C (2005) Genetic deletion of the p66Shc adaptor protein protects from angiotensin II-induced myocardial damage. Hypertension 46:433–440
Gutierrez J, Ballinger SW, Darley-Usmar VM, Landar A (2006) Free radicals, mitochondria, and oxidized lipids: the emerging role in signal transduction in vascular cells. Circ Res 99:924–932
Haynes V, Elfering SL, Squires RJ, Traaseth N, Solien J, Ettl A, Giulivi C (2003) Mitochondrial nitric-oxide synthase: role in pathophysiology. IUBMB Life 55:599–603
Hengartner MO (2000) The biochemistry of apoptosis. Nature 407:770–776
Ide T, Tsutsui H, Kinugawa S, Suematsu N, Hayashidani S, Ichikawa K, Utsumi H, Machida Y, Egashira K, Takeshita A (2000) Direct evidence for increased hydroxyl radicals originating from superoxide in the failing myocardium. Circ Res 86:152–157
Janssen-Heininger YM, Mossman BT, Heintz NH, Forman HJ, Kalyanaraman B, Finkel T, Stamler JS, Rhee SG, van d, V (2008) Redox-based regulation of signal transduction: principles, pitfalls, and promises. Free Radic Biol Med 45:1–17
Kaludercic N, Feng N, Nagayama T, Bedja D, Carpi A, Vecoli C, Cormaci G, Gabrielson K, Kass D, Paolocci N, Di Lisa F (2007) Monoamine oxidase A is upregulated in cardiac hypertrophy and is a major determinant of the transition from compensation to failure. Circ Res 101(11):7 (abstract)
Lemasters JJ, Qian T, Bradham CA, Brenner DA, Cascio WE, Trost LC, Nishimura Y, Nieminen AL, Herman B (1999) Mitochondrial dysfunction in the pathogenesis of necrotic and apoptotic cell death. J Bioenerg Biomembr 31:305–319
Lenders JW, Eisenhofer G, Abeling NG, Berger W, Murphy DL, Konings CH, Wagemakers LM, Kopin IJ, Karoum F, van Gennip AH, Brunner HG (1996) Specific genetic deficiencies of the A and B isoenzymes of monoamine oxidase are characterized by distinct neurochemical and clinical phenotypes. J Clin Invest 97:1010–1019
Levitt P, Pintar JE, Breakefield XO (1982) Immunocytochemical demonstration of monoamine oxidase B in brain astrocytes and serotonergic neurons. Proc Natl Acad Sci USA 79:6385–6389
Liu Y, Yang XM, Iliodromitis EK, Kremastinos DT, Dost T, Cohen MV, Downey JM (2008) Redox signaling at reperfusion is required for protection from ischemic preconditioning but not from a direct PKC activator. Basic Res Cardiol 103:54–59
Madamanchi NR, Runge MS (2007) Mitochondrial dysfunction in atherosclerosis. Circ Res 100:460–473
Maurel A, Hernandez C, Kunduzova O, Bompart G, Cambon C, Parini A, Frances B (2003) Age-dependent increase in hydrogen peroxide production by cardiac monoamine oxidase A in rats. Am J Physiol Heart Circ Physiol 284:H1460–H1467
Melov S, Coskun PE, Wallace DC (1999) Mouse models of mitochondrial disease, oxidative stress, and senescence. Mutat Res 434:233–242
Menini S, Amadio L, Oddi G, Ricci C, Pesce C, Pugliese F, Giorgio M, Migliaccio E, Pelicci P, Iacobini C, Pugliese G (2006) Deletion of p66Shc longevity gene protects against experimental diabetic glomerulopathy by preventing diabetes-induced oxidative stress. Diabetes 55:1642–1650
Migliaccio E, Giorgio M, Mele S, Pelicci G, Reboldi P, Pandolfi PP, Lanfrancone L, Pelicci PG (1999) The p66Shc adaptor protein controls oxidative stress response and life span in mammals. Nature 402:309–313
Migliaccio E, Giorgio M, Pelicci PG (2006) Apoptosis and aging: role of p66Shc redox protein. Antioxid Redox Signal 8:600–608
Murphy MP (2009) How mitochondria produce reactive oxygen species. Biochem J (in press)
Murphy E, Steenbergen C (2008) Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev 88:581–609
Napoli C, Martin-Padura I, de Nigris F, Giorgio M, Mansueto G, Somma P, Condorelli M, Sica G, De Rosa G, Pelicci P (2003) Deletion of the p66Shc longevity gene reduces systemic and tissue oxidative stress, vascular cell apoptosis, and early atherogenesis in mice fed a high-fat diet. Proc Natl Acad Sci USA 100:2112–2116
Nemoto S, Finkel T (2002) Redox regulation of forkhead proteins through a p66Shc-dependent signaling pathway. Science 295:2450–2452
Newmeyer DD, Ferguson-Miller S (2003) Mitochondria: releasing power for life and unleashing the machineries of death. Cell 112:481–490
Ohashi M, Runge MS, Faraci FM, Heistad DD (2006) MnSOD deficiency increases endothelial dysfunction in ApoE-deficient mice. Arterioscler Thromb Vasc Biol 26:2331–2336
Okado-Matsumoto A, Fridovich I (2001) Subcellular distribution of superoxide dismutases (SOD) in rat liver: Cu, Zn-SOD in mitochondria. J Biol Chem 276:38388–38393
Orsini F, Migliaccio E, Moroni M, Contursi C, Raker VA, Piccini D, Martin-Padura I, Pelliccia G, Trinei M, Bono M, Puri C, Tacchetti C, Ferrini M, Mannucci R, Nicoletti I, Lanfrancone L, Giorgio M, Pelicci PG (2004) The life span determinant p66Shc localizes to mitochondria where it associates with mitochondrial heat shock protein 70 and regulates trans-membrane potential. J Biol Chem 279:25689–25695
Papa S, Skulachev VP (1997) Reactive oxygen species, mitochondria, apoptosis and aging. Mol Cell Biochem 174:305–319
Pchejetski D, Kunduzova O, Dayon A, Calise D, Seguelas MH, Leducq N, Seif I, Parini A, Cuvillier O (2007) Oxidative stress-dependent sphingosine kinase-1 inhibition mediates monoamine oxidase A-associated cardiac cell apoptosis. Circ Res 100:41–49
Pelicci G, Lanfrancone L, Grignani F, McGlade J, Cavallo F, Forni G, Nicoletti I, Grignani F, Pawson T, Pelicci PG (1992) A novel transforming protein (SHC) with an SH2 domain is implicated in mitogenic signal transduction. Cell 70:93–104
Pellegrini M, Pacini S, Baldari CT (2005) p66Shc: the apoptotic side of Shc proteins. Apoptosis 10:13–18
Pinton P, Rimessi A, Marchi S, Orsini F, Migliaccio E, Giorgio M, Contursi C, Minucci S, Mantovani F, Wieckowski MR, Del SG, Pelicci PG, Rizzuto R (2007) Protein kinase C beta and prolyl isomerase 1 regulate mitochondrial effects of the life-span determinant p66Shc. Science 315:659–663
Pletscher A (1991) The discovery of antidepressants: a winding path. Experientia 47:4–8
Poderoso JJ, Carreras MC, Lisdero C, Riobo N, Schopfer F, Boveris A (1996) Nitric oxide inhibits electron transfer and increases superoxide radical production in rat heart mitochondria and submitochondrial particles. Arch Biochem Biophys 328:85–92
Purdom S, Chen QM (2003) p66(Shc): at the crossroad of oxidative stress and the genetics of aging. Trends Mol Med 9:206–210
Riederer P, Lachenmayer L, Laux G (2004) Clinical applications of MAO-inhibitors. Curr Med Chem 11:2033–2043
Rota M, LeCapitaine N, Hosoda T, Boni A, De AA, Padin-Iruegas ME, Esposito G, Vitale S, Urbanek K, Casarsa C, Giorgio M, Luscher TF, Pelicci PG, Anversa P, Leri A, Kajstura J (2006) Diabetes promotes cardiac stem cell aging and heart failure, which are prevented by deletion of the p66Shc gene. Circ Res 99:42–52
Salet C, Moreno G, Ricchelli F, Bernardi P (1997) Singlet oxygen produced by photodynamic action causes inactivation of the mitochondrial permeability transition pore. J Biol Chem 272:21938–21943
Sarkela TM, Berthiaume J, Elfering S, Gybina AA, Giulivi C (2001) The modulation of oxygen radical production by nitric oxide in mitochondria. J Biol Chem 276:6945–6949
Shih JC (2004) Cloning, after cloning, knock-out mice, and physiological functions of MAO A and B. Neurotoxicology 25:21–30
Siddall HK, Warrell CE, Yellon DM, Mocanu MM (2008) Ischemia-reperfusion injury and cardioprotection: investigating PTEN, the phosphatase that negatively regulates PI3 K, using a congenital model of PTEN haploinsufficiency. Basic Res Cardiol 103:560–568
Sivasubramaniam SD, Finch CC, Rodriguez MJ, Mahy N, Billett EE (2003) A comparative study of the expression of monoamine oxidase-A and -B mRNA and protein in non-CNS human tissues. Cell Tissue Res 313:291–300
Tipton KF, Boyce S, O’Sullivan J, Davey GP, Healy J (2004) Monoamine oxidases: certainties and uncertainties. Curr Med Chem 11:1965–1982
Trinei M, Giorgio M, Cicalese A, Barozzi S, Ventura A, Migliaccio E, Milia E, Padura IM, Raker VA, Maccarana M, Petronilli V, Minucci S, Bernardi P, Lanfrancone L, Pelicci PG (2002) A p53–p66Shc signalling pathway controls intracellular redox status, levels of oxidation-damaged DNA and oxidative stress-induced apoptosis. Oncogene 21:3872–3878
Turrens JF (2003) Mitochondrial formation of reactive oxygen species. J Physiol 552:335–344
Wenzel P, Muller J, Zurmeyer S, Schuhmacher S, Schulz E, Oelze M, Pautz A, Kawamoto T, Wojnowski L, Kleinert H, Munzel T, Daiber A (2008) ALDH-2 deficiency increases cardiovascular oxidative stress–evidence for indirect antioxidative properties. Biochem Biophys Res Commun 367:137–143
Youdim MB, Edmondson D, Tipton KF (2006) The therapeutic potential of monoamine oxidase inhibitors. Nat Rev Neurosci 7:295–309
Zaccagnini G, Martelli F, Fasanaro P, Magenta A, Gaetano C, Di Carlo A, Biglioli P, Giorgio M, Martin-Padura I, Pelicci PG, Capogrossi MC (2004) p66ShcA modulates tissue response to hindlimb ischemia. Circulation 109:2917–2923
Zhao W, Fan GC, Zhang ZG, Bandyopadhyay A, Zhou X, Kranias EG (2008) Protection of peroxiredoxin II on oxidative stress-induced cardiomyocyte death and apoptosis. Basic Res Cardiol (in press)
Acknowledgments
This work has been supported by University of Padova (Post-Doctoral Fellowship to NK), MIUR and CNR.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Di Lisa, F., Kaludercic, N., Carpi, A. et al. Mitochondrial pathways for ROS formation and myocardial injury: the relevance of p66Shc and monoamine oxidase. Basic Res Cardiol 104, 131–139 (2009). https://doi.org/10.1007/s00395-009-0008-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00395-009-0008-4