Abstract
Aim
During atrial fibrillation, arterial hypertension and systolic or diastolic heart failure, atrial myocytes are exposed to increased baseline stretch. Atrial stretch has been shown to induce cellular hypertrophy and extracellular matrix remodeling (ECM) via angiotensin-II dependent pathways and the matrix metalloproteinases system (MMPs). We hypothesized that atrial myocytes exposed to static stretch may increase their ECM remodeling activity via up-regulation of MMP-2/-9. We then tested the hypothesis that the membrane bound angiotensin-II type 1 (AT1) receptor and the intracellular calcineurin (Cn)-NFAT signaling pathway are potential mediators of stretch-induced MMP alterations, since Cn-NFAT is one important contributor to myocyte hypertrophy.
Methods and results
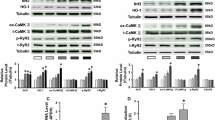
Neonatal rat atrial myocytes (NRAM) were cultured under conditions of static stretch by 21%. The differential effects of selective AT1 receptor blockade by losartan, Cn blockade by Cyclosporine-A (CsA) or NFAT inhibition by 11R-VIVIT (VIV), were analyzed. Stretch resulted in a significant up-regulation of active-MMP-2/-9 protein amount (active-MMP-2 ng/µg: control 8.95 ± 0.64 vs. stretch 13.11 ± 0.74 / active-MMP-9 ng/µg: control 1.45 ± 0.18 vs. stretch 1.94 ± 0.21, all n = 5) and enzyme activity (MMP-2 in %: control 1 ± 0.0 vs. stretch 1.87 ± 0.25, n = 7) associated with a significant increase of the membrane-type-1-MMP (MT1-MMP) protein expression (MT1-MMP in %: control 1 ± 0.0 vs. stretch 2.17 ± 0.21, n = 8). These observations were accompanied by an activation of the Cn-NFAT pathway (Cn-activity in nmol PO4 release/20 µg protein/30 min: control 0.37 ± 0.08 vs. stretch 0.65 ± 0.09, n = 3 / NFATc1-DNA binding activity in %: control 1 ± 0.0 vs. stretch 1.53 ± 0.17, n = 3). Losartan, CsA or VIV abolished stretch-induced alterations in MMP-2/-9 and MT1-MMP expression and enzyme activity by normalizing the Cn-activity and the DNA binding activity of NFATc1.
Conclusion
Our results present new insights in molecular mechanisms of ECM remodeling activity of atrial myocytes exposed to static stretch. The AT1-Cn-NFAT pathway is a potential mediator of MMP activation.





Similar content being viewed by others
References
Allessie M, Ausma J, Schotten U (2002) Electrical, contractile and structural remodeling during atrial fibrillation. Cardiovasc Res 54:230–246
Aramburu J, Yaffe MB, Lopez-Rodriguez C, Cantley LC, Hogan PG, Rao A (1999) Affinity-driven peptide selection of an NFAT inhibitor more selective than Cyclosporin A. Science 285:2129–2133
Bukowska A, Lendeckel U, Hirte D, Wolke C, Striggow F, Röhnert P, Huth C, Klein HU, Goette A (2006) Activation of the calcineurin signaling pathway induces atrial hypertrophy during atrial fibrillation. Cell Mol Life Sci 63:333–342
Fabunmi RP, Baker AH, Murray EJ, Booth RFG, Newby AC (1996) Divergent regulation by growth factors and cytokines of 95 kDa and 72 kDa gelatinases and tissue inhibitors of metalloproteinases-1, -2, and -3 in rabbit aortic smooth muscle cells. Biochem J 315:335-342
Gallagher G, Menzie S, Huang Y, Jackson C, Hunyor SN (2007) Regional cardiac dysfunction is associated with specific alterations in inflammatory cytokines and matrix metalloproteinases after acute myocardial infarction in sheep. Basic Res Cardiol 102:63–72
Goette A, Staack T, Röcken C, Arndt M, Geller JC, Huth C, Ansorge S, Klein HU, Lendeckel U (2000) Increased expression of extracellular signal-regulated kinase and angiotensin-converting enzyme in human atria during atrial fibrillation. J Am Coll Cardiol 35:1669–1677
Gupta V, Grande-Allen KJ (2006) Effects of static and cyclic loading in regulating extracellular matrix synthesis by cardiovascular cells. Cardiovasc Res 72:375–383
Jaïs P, Peng JT, Shah DC, Garrigue S, Hocini M, Yamane T, Haïssaguerre M, Barold SS, Roudaut R, Clémenty J (2000) Left ventricular diastolic dysfunction in patients with so-called lone atrial fibrillation. J Cardiovasc Electrophysiol 11:623–625
Kannel WB, Abbott RD, Savage DD, McNamara PM (1982) Epidemiologic features of atrial fibrillation. N Engl J Med 306:1018–1022
Kleiner DE, Stetler-Stevenson WG (1994) Quantitative zymography: detection of picogram quantities of gelatinases. Anal Biochem 218:325–329
Li D, Fareh S, Leung TL, Nattel S (1999) Promotion of atrial fibrillation by heart failure in dogs. Atrial remodeling of a different sort. Circulation 100:87–95
Li Y, Li WM, Gong YT, Li BX, Liu W, Han W, Dong D, Sheng L, Xue JY, Zhang L, Chu S, Yang BF (2007) The effects of cilazapril and valsartan on the mRNA and protein expressions of atrial calpains and atrial structural remodeling in atrial fibrillation dogs. Basic Res Cardiol 102:245–256
Lin CC, Lin JL, Lin CS, Tsai MC, Su MJ, Lai LP, Huang SK (2004) Activation of the calcineurin-nuclear factor pathway of activated T-cell signal transduction in atrial fibrillation. Chest 126:1926–1932
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–408
Malhotra R, Sadoshima J, Brosius FC, Izumo S (1999) Mechanical stretch and angiotensin-II differentially upregulate the renin-angiotensin system in cardiac myocytes in vitro. Circ Res 85:137–146
McEwan PE, Sherry L, Kenyon CJ, Webb DJ, Gray GA (2000) Regulation of the myocardial endothelin system by angiotensin-II and losartan. J Cardiovasc Pharmacol 36 (5 suppl 1):S144–S147
Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, Olson EN (1998) A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell 93:215–228
Monea S, Lehti K, Keski-Oja J, Mignatti P (2002) Plasmin activates pro-matrix metalloproteinase-2 with a membrane-type 1 matrix metalloproteinase-dependent mechanism. J Cell Physiol 192:160–170
Nagata K, Somura F, Obata K, Odashima M, Izawa H, Ichihara S, Nagasaka T, Iwase M, Yamada Y, Nakashima N, Yokota M (2002) AT1 receptor blockade reduces cardiac calcineurin activity in hypertensive rats. Hypertension 40:168–174
Pérez NG, de Hurtado MC, Cingolani HE (2001) Reverse mode of the Na+–Ca2+ exchange after myocardial stretch: underlying mechanism of the slow force response. Circ Res 88:376–382
Poenicke K, Heinroth-Hoffmann I, Becker K, Brodde OE (1997) Trophic effect of angiotensin-II in neonatal rat cardiomyocytes: role of endothelin-1 and non-myocyte cells. Br J Pharmacol 121:118–124
Polontchouk L, Ebelt B, Jackels M, Dhein S (2001) Chronic effects of endothelin 1 and angiotensin-II on gap junctions and intercellular communication in cardiac cells. FASEB J 16:87–89
Polyakova V, Hein S, Kostina S, Ziegelhoeffer T, Schaper J (2004) Matrix metalloproteinases and their tissue inhibitors in pressure-overloaded human myocardium during heart failure progression. J Am Coll Cardiol 44:1609–1618
Polyakova V, Miyagawa S, Szalay Z, Risteli J, Kostina S (2008) Atrial extracellular matrix remodelling in patients with atrial fibrillation. J Cell Mol Med 12:189–208
Psaty BM, Manolio TA, Kuller LH, Kronmal RA, Cushman M, Fried LP, White R, Furberg CD, Rautaharju PM (1997) Incidence of and risk factors for atrial fibrillation in older adults. Circulation 96:2455–2461
Rana OR, Zobel C, Saygili E, Brixius K, Gramley F, Schimpf T, Mischke K, Frechen D, Knackstedt Ch, Schwinger RHG, Schauerte P, Saygili E (2008) A simple device to apply equibiaxial strain to cells cultured on flexible membranes. Am J Physiol Heart Circ Physiol 294:H532–H540
Ries C, Petrides PE (1995) Cytokine regulation of matrix metalloproteinase activity and its regulatory dysfunction in disease. Biol Chem 376:345–355
Rossi GP, Sacchetto A, Cesari M, Pessina AC (1999) Interactions between endothelin-1 and the renin–angiotensin–aldosterone system. Cardiovasc Res 43:300–307
Sadoshima J, Xu Y, Slayter HS, Izumo S (1993) Autocrine release of angiotensin-II mediates stretch-induced hypertrophy of cardiac myocytes in vitro. Cell 75:977–984
Saygili E, Rana OR, Saygili E, Reuter H, Frank KF, Schwinger RH, Muller-Ehmsen J, Zobel C (2007) Losartan prevents stretch induced electrical remodeling in cultured atrial neonatal myocytes. Am J Physiol Heart Circ Physiol 292:H2898–H2905
Schott P, Asif AR, Gräf C, Toischer K, Hasenfuss G, Kögler H (2008) Myocardial adaptation of energy metabolism to elevated preload depends on calcineurin activity: a proteomic approach. Basic Res Cardiol 103:232–243
Seeland U, Selejan S, Engelhardt S, Müller P, Lohse MJ, Böhm M (2008) Interstitial remodeling in beta1-adrenergic receptor transgenic mice. Basic Res Cardiol 102:183–193
Verheule S, Wilson E, Everett T 4th, Shanbhag S, Golden C, Olgin J (2003) Alterations in atrial electrophysiology and tissue structure in a canine model of chronic atrial dilatation due to mitral regurgitation. Circulation 107:2615–2622
Vermes E, Tardif JC, Bourassa MG, Racine N, Levesque S, White M, Guerra PG, Ducharme A (2003) Enalapril decreases the incidence of atrial fibrillation in patients with left ventricular dysfunction: insight from the studies of left ventricular dysfunction (SOLVD) trials. Circulation 107:2926–2931
Wachtell K, Lehto M, Gerdts E, Olsen MH, Hornestam B, Dahlof B, Ibsen H, Julius S, Kjeldsen SE, Lindholm LH, Nieminen MS, Devereux RB (2005) Angiotensin-II receptor blockade reduces new-onset atrial fibrillation and subsequent stroke compared to atenolol: the losartan intervention for end point reduction in hypertension (LIFE) study. J Am Coll Cardiol 45:712–719
Wang TL, Yang YH, Chang H, Hung CR (2004) Angiotensin-II signals mechanical stretch-induced cardiac matrix metalloproteinase expression via JAK-STAT pathway. J Mol Cell Cardiol 37:785–794
Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA (1995) Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation 92:1954–1968
Wilkins BJ, Windt LJ, Bueno OF, Braz JC, Glascock BJ, Kimbal TF, Molkentin JD (2002) Targeted disruption of NFATc3, but not NFATc4, reveals an intrinsic defect in calcineurin mediated cardiac hypertrophic growth. Mol Cell Biol 22:7603–7613
Will H, Atkinson SJ, Butler GS, Smith B, Murphy G (1996) The soluble catalytic domain of membrane type 1 matrix metalloproteinase cleaves the propeptide of progelatinase A and initiate autoproteolytic activation: regulation by TIMP 2 and TIMP 3. J Biol Chem 271:17119–17123
Yamamoto K, Dang QN, Kelly RA, Lee RT (1998) Mechanical strain suppresses inducible nitric-oxide synthase in cardiac myocytes. J Biol Chem 273:11862–11866
Zobel C, Rana OR, Saygili E, Bölk B, Saygili E, Diedrichs H, Reuter H, Frank K, Müller-Ehmsen J, Pfitzer G, Schwinger RHG (2007) Mechanisms of Ca2+-dependent calcineurin activation in mechanical stretch-induced hypertrophy. Cardiology 107:281–290
Acknowledgments
We thank the entire Institute of Laboratory Animal Science, University Hospital, RWTH Aachen in Germany for the helpful assistance in animal research. This work was supported in part by Deutsche Forschungsgemeinschaft (DFG) grant Lu 869/4-1 and IZKF Biomat. RWTH Aachen, Germany to A.L. and by the Network of Competence Atrial Fibrillation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Returned for 1. Revision: 15 August 2008 1. Revision received: 17 November 2008
E. Saygili and O. R. Rana contributed equally to this work.
Rights and permissions
About this article
Cite this article
Saygili, E., Rana, O.R., Meyer, C. et al. The angiotensin–calcineurin–NFAT pathway mediates stretch-induced up-regulation of matrix metalloproteinases-2/-9 in atrial myocytes. Basic Res Cardiol 104, 435–448 (2009). https://doi.org/10.1007/s00395-008-0772-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00395-008-0772-6