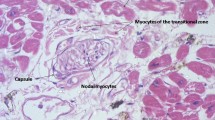
Abstract
Objective
We studied the response of the superior vena cava (SVC) myocardial sleeve to atrial fibrillation (AF).
Methods and results
We examined adult male dogs without pacing (N=6) and after rapid atrial pacing (600 bpm) for 2 weeks (P2w; N=5) and 6–8 weeks (P6–8w; N=5). After pacing, the sleeve was increased in thickness (non-paced vs. either paced group, both P<0.05). This was associated with an increase in proliferative activity, which was higher in the P2w than the P6–8w animals (P < 0.05). In addition, collagen content increased, and the component cardiomyocytes become more unevenly oriented and shorter and narrower in shape (non-paced vs. either paced group, both P < 0.05). Pacing had different effects on connexin40 (Cx40) and Cx43 gap junctions. There was a 98% increase in Cx43 signal in P2w, and a 74% increase in P6–8w animals (non-paced vs. each paced group, both P < 0.05). In contrast, Cx40 signal decreased 47% in P2w but increased 44% in P6–8w animals (non-paced vs. each paced group, both P < 0.05).
Conclusions
Rapid atrial pacing results in a specific pattern of remodeling of the canine SVC sleeve, including changes in size and shape, spatial orientation, and gap junction expression profile of the component cardiomyocytes. These changes may co-operatively affect the electrical properties and contribute to the formation and maintenance of the arrhythmogenic substrate of AF.







Similar content being viewed by others
References
Tsai CF, Tai CT, Hsieh MH, Lin WS, Yu WC, Ueng KC, Ding YA, Chang MS, Chen SA (2000) Initiation of atrial fibrillation by ectopic beats originating from the superior vena cava: electrophysiological characteristics and results of radiofrequency ablation. Circulation 102:67–74
Lu TM, Tai CT, Hsieh MH, Tsai CF, Lin YK, Yu WC, Tsao HM, Lee SH, Ding YA, Chang MS, Chen SA (2001) Electrophysiologic characteristics in initiation of paroxysmal atrial fibrillation from a focal area. J Am Coll Cardiol 37:1658–1664
Hayashi H, Wang C, Miyauchi Y, Omichi C, Pak HN, Zhou S, Ohara T, Mandel WJ, Lin SF, Fishbein MC, Chen PS, Karagueuzian HS (2002) Aging-related increase to inducible atrial fibrillation in the rat model. J Cardiovasc Electrophysiol 13:801–808
Ausma J, van der Velden HM, Lenders MH, van Ankeren EP, Jongsma HJ, Ramaekers FC, Borgers M, Allessie MA (2003) Reverse structural and gap-junctional remodeling after prolonged atrial fibrillation in the goat. Circulation 107:2051–2058
Ausma J, Litjens N, Lenders MH, Duimel H, Mast F, Wouters L, Ramaekers F, Allessie M, Borgers M (2001) Time course of atrial fibrillation-induced cellular structural remodeling in atria of the goat. J Mol Cell Cardiol 33:2083–2094
Lee SH, Chen YJ, Tai CT, Yeh HI, Cheng JJ, Hung CR, Chen SA (2005) Electrical remodeling of the canine superior vena cava after chronic rapid atrial pacing. Basic Res Cardiol 100:14–21
Severs NJ, Coppen SR, Dupont E, Yeh HI, Ko YS, Matsushita T (2004) Gap junction alterations in human cardiac disease. Cardiovasc Res 62:368–377
van der Velden HM, Jongsma HJ (2002) Cardiac gap junctions and connexins: their role in atrial fibrillation and potential as therapeutic targets. Cardiovasc Res 54:270–279
Yeh HI, Lai YJ, Lee SH, Lee YN, Ko YS, Chen SA, Severs NJ, Tsai CH (2001) Heterogeneity of myocardial sleeve morphology and gap junctions in canine superior vena cava. Circulation 104:3152–3157
Yeh HI, Lai YJ, Lee YN, Chen YJ, Chen YC, Chen CC, Chen SA, Lin CI, Tsai CH (2003) Differential expression of connexin43 gap junctions in cardiomyocytes isolated from canine thoracic veins. J Histochem Cytochem 51:259–266
Kostin S, Klein G, Szalay Z, Hein S, Bauer EP, Schaper J (2002) Structural correlate of atrial fibrillation in human patients. Cardiovasc Res 54:361–379
Nao T, Ohkusa T, Hisamatsu Y, Inoue N, Matsumoto T, Yamada J, Shimizu A, Yoshiga Y, Yamagata T, Kobayashi S, Yano M, Hamano K, Matsuzaki M (2003) Comparison of expression of connexin in right atrial myocardium in patients with chronic atrial fibrillation versus those in sinus rhythm. Am J Cardiol 91:678–683
Polontchouk L, Haefliger JA, Ebelt B, Schaefer T, Stuhlmann D, Mehlhorn U, Kuhn-Regnier F, De Vivie ER, Dhein S (2001) Effects of chronic atrial fibrillation on gap junction distribution in human and rat atria. J Am Coll Cardiol 38:883–891
Elvan A, Huang XD, Pressler ML, Zipes DP (1997) Radiofrequency catheter ablation of the atria eliminates pacing-induced sustained atrial fibrillation and reduces connexin 43 in dogs. Circulation 96:1675–1685
van der Velden HM, van Kempen MJ, Wijffels MC, van Zijverden M, Groenewegen WA, Allessie MA, Jongsma HJ (1998) Altered pattern of connexin40 distribution in persistent atrial fibrillation in the goat. J Cardiovasc Electrophysiol 9:596–607
Lee SH, Lin FY, Yu WC, Cheng JJ, Kuan P, Hung CR, Chang MS, Chen SA (1999) Regional differences in the recovery course of tachycardia-induced changes of atrial electrophysiological properties. Circulation 99:1255–1264
Thijssen VL, Ausma J, Liu GS, Allessie MA, van Eys GJ, Borgers M (2000) Structural changes of atrial myocardium during chronic atrial fibrillation. Cardiovasc Pathol 9:17–28
Allessie M, Ausma J, Schotten U (2002) Electrical, contractile and structural remodeling during atrial fibrillation. Cardiovasc Res 54:230–246
Bauer A, McDonald AD, Donahue JK (2004) Pathophysiological findings in a model of persistent atrial fibrillation and severe congestive heart failure. Cardiovasc Res 61:764–770
Everett TH, Li H, Mangrum JM, McRury ID, Mitchell MA, Redick JA, Haines DE (2000) Electrical, morphological, and ultrastructural remodeling and reverse remodeling in a canine model of chronic atrial fibrillation. Circulation 102:1454–1460
Hassink RJ, Aretz HT, Ruskin J, Keane D (2003) Morphology of atrial myocardium in human pulmonary veins: a postmortem analysis in patients with and without atrial fibrillation. J Am Coll Cardiol 42:1108–1114
Liu Y, Cigola E, Cheng W, Kajstura J, Olivetti G, Hintze TH, Anversa P (1995) Myocyte nuclear mitotic division and programmed myocyte cell death characterize the cardiac myopathy induced by rapid ventricular pacing in dogs. Lab Invest 73:771–787
Mary-Rabine L, Albert A, Pham TD, Hordof A, Fenoglio JJ Jr, Malm JR, Rosen MR (1983) The relationship of human atrial cellular electrophysiology to clinical function and ultrastructure. Circ Res 52:188–199
van der Velden HM, Ausma J, Rook MB, Hellemons AJ, van Veen TA, Allessie MA, Jongsma HJ (2000) Gap junctional remodeling in relation to stabilization of atrial fibrillation in the goat. Cardiovasc Res 46:476–486
Spach MS, Heidlage JF, Dolber PC, Barr RC (2000) Electrophysiological effects of remodeling cardiac gap junctions and cell size: experimental and model studies of normal cardiac growth. Circ Res 86:302–311
Jongsma HJ, Wilders R (2000) Gap junctions in cardiovascular disease. Circ Res 86:1193–1197
Kleber AG (1999) Discontinuous propagation of the cardiac impulse and arrhythmogenesis. J Cardiovasc Electrophysiol 10:1025–1027
Acknowledgments
Supported from the National Science Council, Taiwan (NSC-92-2314-B-195-012 and 93-2314-B-195-019) and from the Medical Research Department of the Mackay Memorial Hospital, Taiwan (MMH-E 94003). The authors thank miss Nai-Feng Cheng’s contribution in assistance of sample preparation in this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yeh, HI., Lai, YJ., Lee, SH. et al. Remodeling of myocardial sleeve and gap junctions in canine superior vena cava after rapid pacing. Basic Res Cardiol 101, 269–280 (2006). https://doi.org/10.1007/s00395-006-0588-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00395-006-0588-1