Abstract
Background
Myocardial stress and strain are considered primary mechanical stimuli for hypertrophic remodeling. Their values and signifi– cance in the intact beating heart during chronic overload remain poorly characterized.
Methods and results
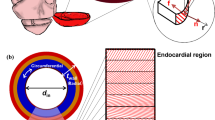
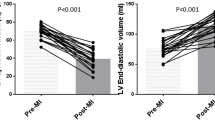
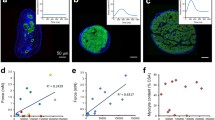
Left–ventricular (LV) dimensions (echocardiography) and pressure (invasive) were simultaneously recorded in anesthetized dogs at sinus rhythm (SR), acute and 1, 2, 6, 12 weeks of atrioventricular block (AVB), leading to structural, electrical and contractile remodeling. Mechanical load of the myocardium was quantified as myofiber stress (σf), being force along myofiber orientation per cross–sectional area, and natural myofiber strain (ef), being change in natural logarithm of myofiber length (l) divided by its reference length: ef = ln(l/lref). Time courses of σf and ef were calculated from LV pressure and dimensions, using a validated mathematical model of cardiac mechanics. End–diastolic σf increased from 2.0 ± 0.1 kPa at SR to 3.4 ± 0.3 kPa at acute AVB, remaining elevated for > 6 weeks. Systolic σf was not affected by AVB. Ejection strain rose instantly upon AVB, reaching a maximum at 2 weeks: 0.24 ± 0.02 vs. 0.10 ± 0.01 at SR. The increase of myofiber stroke work (σf–ef loop area) from 3.1 ± 0.3 at SR to 6.0 ± 0.5 kJ/m3/beat at 1 week AVB was attributed mainly to an increase of strain during ejection. Stroke work and ejection strain remained elevated up to 12 weeks. The rate of LV–mass increase was maximal (2.2 ± 0.4 g/day) at 1 week AVB.
Conclusions
Serial mechanical phenotyping is feasible in the intact anesthetized dog with chronic ventricular overload. Our new approach yields values of mechanical load that are comparable to those found in isolated myocardium by others. In chronic AVB, both end–diastolic myofiber stress and ejection strain are increased. Early increases of both parameters coincide with peak hypertrophic growth, suggesting their important role for mechanotransduction. Peak systolic σf is likely not important for hypertrophy in this model, since it does not change throughout the experiment.
Similar content being viewed by others
References
Arts T, Bovendeerd PH, Prinzen FW et al. (1991) Relation between left ventricular cavity pressure and volume and systolic fiber stress and strain in the wall. Biophys J 59:93–102
Ashikaga H, Covell JW, Omens JH (2005) Diastolic dysfunction in volume overload hypertrophy is associated with abnormal shearing of myolaminar sheets. Am J Physiol Heart Circ Physiol (Epub ahead of print)
de Tombe PP, ter Keurs HE (1991) Sarcomere dynamics in cat cardiac trabeculae. Circ Res 68:588–596
Delhaas T, Arts T, Bovendeerd PH et al. (1993) Subepicardial fiber strain and stress as related to left ventricular pressure and volume. Am J Physiol 264:H1548–H1559
Delhaas T, Arts T, Prinzen FW et al. (1998) Estimates of regional work in the canine left ventricle. Prog Biophys Mol Biol 69:273–287
Falsetti HL, Mates RE, Grant C et al. (1970) Left ventricular wall stress calculated from one–plane cineangiography. Circ Res 26:71–83
Florenzano F, Glantz SA (1987) Left ventricular mechanical adaptation to chronic aortic regurgitation in intact dogs. Am J Physiol 252:H969–H984
Granzier HL, Irving TC (1995) Passive tension in cardiac muscle: contribution of collagen, titin, microtubules, and intermediate filaments. Biophys J 68:1027–1044
Grossman W, Jones D, McLaurin LP (1975) Wall stress and patterns of hypertrophy in the human left ventricle. J Clin Invest 56:56–64
Herbots L, Maes F, D’Hooge J et al. (2004) Quantifying myocardial deformation throughout the cardiac cycle: a comparison of ultrasound strain rate, grey–scale M–mode and magnetic resonance imaging. Ultrasound Med Biol 30:591–598
Holmes JW (2004) Candidate mechanical stimuli for hypertrophy during volume overload. J Appl Physiol 97:1453–1460
Hu Z, Metaxas D, Axel L (2003) In vivo strain and stress estimation of the heart left and right ventricles from MRI images. Med Image Anal 7:435–444
Hunter JJ, Chien KR (1999) Signaling pathways for cardiac hypertrophy and failure. N Engl J Med 341:1276–1283
Kerckhoffs RC, Bovendeerd PH, Kotte JC et al. (2003) Homogeneity of cardiac contraction despite physiological asynchrony of depolarization: a model study. Ann Biomed Eng 31:536–547
Komuro I, Kaida T, Shibazaki Y et al. (1990) Stretching cardiac myocytes stimulates protooncogene expression. J Biol Chem 265:3595–3598
Mirsky I (1973) Ventricular and arterial wall stresses based on large deformation analyses. Biophys J 13:1141–1159
Miyamoto T, Takeishi Y, Takahashi H et al. (2004) Activation of distinct signal transduction pathways in hypertrophied hearts by pressure and volume overload. Basic Res Cardiol 99:328–337
Modesti PA, Vanni S, Bertolozzi I et al. (2000) Early sequence of cardiac adaptations and growth factor formation in pressure– and volume–overload hypertrophy. Am J Physiol Heart Circ Physiol 279:H976–H985
Moustakidis P, Cupps BP, Pomerantz BJ et al. (2004) Noninvasive, quantitative assessment of left ventricular function in ischemic cardiomyopathy. J Surg Res 116:187–196
Nomura S (1986) Diastolic property of left ventricle under experimental volume overload. Jpn Circ J 50:426–432
Omens JH (1998) Stress and strain as regulators of myocardial growth. Prog Biophys Mol Biol 69:559–572
Omens JH, Covell JW (1991) Transmural distribution of myocardial tissue growth induced by volume–overload hypertrophy in the dog. Circulation 84:1235–1245
Reichek N, Helak J, Plappert T et al. (1983) Anatomic validation of left ventricular mass estimates from clinical twodimensional echocardiography: initial results. Circulation 67:348–352
Sadoshima J, Izumo S (1997) The cellular and molecular response of cardiac myocytes to mechanical stress. Annu Rev Physiol 59:551–571
Schreiner KD, Voss F, Senges JC et al. (2004) Tridimensional activation patterns of acquired torsade–de–pointestachycardias in dogs with chronic AVblock. Basic Res Cardiol 99:288–298
Serneri GG, Modesti PA, Boddi M et al. (1999) Cardiac growth factors in human hypertrophy. Relations with myocardial contractility and wall stress. Circ Res 85:57–67
Simpson DG, Majeski M, Borg TK et al. (1999) Regulation of cardiac myocyte protein turnover and myofibrillar structure in vitro by specific directions of stretch. Circ Res 85:e59–e69
Sipido KR, Volders PG, de Groot SH et al. (2000) Enhanced Ca(2+) release and Na/Ca exchange activity in hypertrophied canine ventricular myocytes: potential link between contractile adaptation and arrhythmogenesis. Circulation 102:2137–2144
Snyder DS, Harasawa Y, Sagawa K et al. (1993) Effects of pentobarbital on inotropic state of isolated canine left ventricle. Heart Vessels 8:128–135
Stevens C, Remme E, LeGrice I et al. (2003) Ventricular mechanics in diastole: material parameter sensitivity. J Biomech 36:737–748
van der Toorn A, Barenbrug P, Snoep G et al. (2002) Transmural gradients of cardiac myofiber shortening in aortic valve stenosis patients using MRI tagging. Am J Physiol Heart Circ Physiol 283:H1609–1615
van Opstal JM, Verduyn SC, Leunissen HD et al. (2001) Electrophysiological parameters indicative of sudden cardiac death in the dog with chronic complete AV–block. Cardiovasc Res 50:354–361
Van Trigt P, Christian CC, Fagraeus L et al. (1984) Myocardial depression by anesthetic agents (halothane, enflurane and nitrous oxide): quantitation based on end–systolic pressure–dimension relations. Am J Cardiol 53:243–247
Volders PG, Sipido KR, Vos MA et al. (1998) Cellular basis of biventricular hypertrophy and arrhythmogenesis in dogs with chronic complete atrioventricular block and acquired torsade de pointes. Circulation 98:1136–1147
Vos MA, de Groot SH, Verduyn SC et al. (1998) Enhanced susceptibility for acquired torsade de pointes arrhythmias in the dog with chronic, complete AV block is related to cardiac hypertrophy and electrical remodeling. Circulation 98:1125–1135
Watson PA, Hannan R, Carl LL et al. (1996) Desmin gene expression in cardiac myocytes is responsive to contractile activity and stretch. Am J Physiol 270:C1228–C1235
Wusten B, Flameng W, Winkler B et al. (1977) Role of cardiac contractility in hypertrophy from chronic volume loading. Cardiovasc Res 11:132–140
Yamamoto K, Dang QN, Maeda Y et al. (2001) Regulation of cardiomyocyte mechanotransduction by the cardiac cycle. Circulation 103:1459–1464
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Donker, D.W., Volders, P.G.A., Arts, T. et al. End–diastolic myofiber stress and ejection strain increase with ventricular volume overload. Basic Res Cardiol 100, 372–382 (2005). https://doi.org/10.1007/s00395-005-0525-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00395-005-0525-8