Abstract
Purpose
Ulcerative colitis (UC) is a serious health problem with increasing morbidity and prevalence worldwide. The pathogenesis of UC is complex, currently believed to be influenced by genetic factors, dysregulation of the host immune system, imbalance in the intestinal microbiota, and environmental factors. Currently, UC is typically managed using aminosalicylates, immunosuppressants, and biologics as adjunctive therapies, with the risk of relapse and development of drug resistance upon discontinuation. Therefore, further research into the pathogenesis of UC and exploration of potential treatment strategies are necessary to improve the quality of life for affected patients. According to previous studies, Lactobacillus paracasei Jlus66 (Jlus66) reduced inflammation and may help prevent or treat UC.
Methods
We used dextran sulfate sodium (DSS) to induce a mouse model of UC to assess the effect of Jlus66 on the progression of colitis. During the experiment, we monitored mouse body weight, food and water consumption, as well as rectal bleeding. Hematoxylin-eosin staining was performed to assess intestinal pathological damage. Protein imprinting and immunohistochemical methods were used to evaluate the protein levels of nuclear factor-kappa B (NF-κB), mitogen-activated protein kinase (MAPK), and tight junction (TJ) proteins in intestinal tissues. Fecal microbiota was analyzed based on partial 16S rRNA gene sequencing.
Results
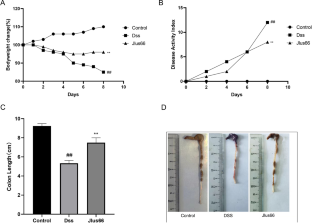
Jlus66 supplementation reduced the degree of colon tissue damage, such as colon shortening, fecal occult blood, colon epithelial damage, and weight loss. Supplementation with Jlus66 reduced DSS-induced upregulation of cytokine levels such as TNF-α, IL-1β, and IL-6 (p < 0.05). The NF-κB pathway and MAPK pathway were inhibited, and the expression of TJ proteins (ZO-1, Occludin, and Claudin-3) was upregulated. 16S rRNA sequencing of mouse cecal contents showed that Jlus66 effectively regulated the structure of the intestinal biota.
Conclusion
In conclusion, these data indicate that Jlus66 can alter the intestinal biota and slow the progression of UC, providing new insights into potential therapeutic strategies for UC.







Similar content being viewed by others
Data availability
The datasets used and analysed in this current study are available from the corresponding author upon reasonable request.
Abbreviations
- UC:
-
Ulcerative Colitis
- DSS:
-
Dextran Sulfate Sodium
- H&E:
-
Haematoxylin and Eosin
- TNF:
-
Tumor Necrosis Factor
- IL:
-
Interleukin
- Gapdh:
-
Glyceraldehyde-3-phosphate dehydrogenase
- CFU:
-
Colony Forming Unit
- AB-PAS:
-
Alcian Blue-Periodic Acid Schif
- NF-κB:
-
Nuclear Factor-Kappa B
- MAPK:
-
Mitogen-Activated Protein Kinase
References
Lee CH et al (2023) Animal models of inflammatory bowel disease: novel experiments for revealing pathogenesis of colitis, fibrosis, and colitis-associated colon cancer. Intestinal Res 21(3):295–305
Wang GQ et al (2019) The ameliorative effect of a strain with good adhesion ability against dextran sulfate sodium-induced murine colitis, vol 10. Food & Function, pp 397–409. 1
Celiberto LS et al (2018) Inflammatory bowel disease and immunonutrition: novel therapeutic approaches through modulation of diet and the gut microbiome. Immunology 155(1):36–52
Shen W, Durum SK (2010) Synergy of IL-23 and Th17 cytokines: New Light on Inflammatory Bowel Disease. Neurochem Res 35(6):940–946
Yue YS et al (2024) Lycopene ameliorated DSS-Induced colitis by improving epithelial barrier functions and inhibiting the Escherichia coli Adhesion in mice. Journal of Agricultural and Food Chemistry
Faith JJ et al (2013) The Long-Term Stability of the human gut microbiota. Science 341(6141):44–
Joo MK et al (2024) Regulation of colonic neuropeptide Y expression by the gut microbiome in patients with ulcerative colitis and its association with anxiety- and depression-like behavior in mice. Gut Microbes, 16(1)
Neufeld SFM et al (2024) Adolescence, the Microbiota-Gut-Brain Axis, and the Emergence of Psychiatric disorders. Biol Psychiatry 95(4):310–318
Xie YS et al (2024) Apple polyphenol extract ameliorates sugary-diet-induced depression-like behaviors in male C57BL/6 mice by inhibiting the inflammation of the gut-brain axis, vol 15. Food & Function, pp 2939–2959. 6
Harris KG, Chang EB (2018) The intestinal microbiota in the pathogenesis of inflammatory bowel diseases: new insights into complex disease. Clin Sci 132(18):2013–2028
Huttenhower C, Kostic AD, Xavier RJ (2014) Inflammatory bowel disease as a model for translating the Microbiome. Immunity 40(6):843–854
Wang C et al (2024) The roles of different strains in alleviating DSS-induced ulcerative colitis and related functional genes. Food & Function
Ao TX et al (2024) Ameliorative effect of bound polyphenols in mung bean coat dietary fiber on DSS-induced ulcerative colitis in mice: the intestinal barrier and intestinal flora. Food & Function
Cheng N et al (2024) Schisandra Chinensis Bee Pollen ameliorates colitis in mice by modulating gut microbiota and regulating Treg/Th17 Balance. Foods, 13(4)
Rocha CS et al (2012) Anti-inflammatory properties of dairy lactobacilli. Inflamm Bowel Dis 18(4):657–666
Hudson LE et al (2017) Gleaning insights from fecal microbiota transplantation and probiotic studies for the Rational Design of Combination Microbial therapies. Clin Microbiol Rev 30(1):191–231
Stofilová J et al (2017) Cytokine production and in rat model of colitis in response to LS/07. Biomed Pharmacother 94:1176–1185
Choi SH et al (2019) CAU1055 ameliorates inflammation in lipopolysaccharide-induced RAW264.7 cells and a dextran sulfate sodium-induced colitis animal model. J Dairy Sci 102(8):6718–6725
Kim MH et al (2018) -derived Extracellular vesicles protect atopic Dermatitis Induced by-derived Extracellular vesicles. Allergy Asthma Immunol Res 10(5–6):516–532
Ye HQ et al (2017) Effect of a novel potential probiotic Jlus66 isolated from fermented milk on nonalcoholic fatty liver in rats. Food Funct 8(12):4539–4546
Ji YY et al (2021) Ameliorates cognitive impairment in high-fat induced obese mice insulin signaling and neuroinflammation pathways, vol 12. Food & Function, pp 8728–8737. 18
Shi K et al (2024) The protective effect of Okanin on colitis induced by dextran sulfate sodium in mice. Food Bioscience, 57
Wang YY et al (2018) Potential of ZDY2013 and WBIN03 in relieving colitis by gut microbiota, immune, and anti-oxidative stress. Can J Microbiol 64(5):327–337
Shi RR et al (2023) Protective effect of subsp. SC-5 on Dextran Sulfate Sodium-Induced Colitis in mice. Foods, 12(4)
Ung V et al (2014) Real-life treatment paradigms Show Infliximab is cost-effective for management of Ulcerative Colitis. Clin Gastroenterol Hepatol 12(11):1871–U351
Hu RJ et al (2021) -derived extracellular vesicles maintain intestinal immune homeostasis against lipopolysaccharide-induced inflammatory responses in broilers. J Anim Sci Biotechnol, 12(1)
Li M et al (2017) -derived extracellular vesicles enhance host immune responses against Vancomycin-resistant enterococci. BMC Microbiol, 17
Schulz-Kuhnt A et al (2024) ATP citrate lyase (ACLY)-dependent immunometabolism in mucosal T cells drives experimental colitis in vivo. Gut
Li C et al (2024) Enocyanin alleviates experimental colitis and restores gut microbiota homeostasis as functional foods. Food Bioscience, 57
Ruffolo C et al (2010) Subclinical intestinal inflammation in patients with Crohn’s Disease following Bowel Resection: a smoldering fire. J Gastrointest Surg 14(1):24–31
Figueredo CM et al (2017) Activity of inflammatory bowel disease influences the expression of cytokines in gingival tissue. Cytokine 95:1–6
Yamamoto-Furusho JK (2018) Inflammatory bowel disease therapy: blockade of cytokines and cytokine signaling pathways. Curr Opin Gastroenterol 34(4):187–193
Kai Y et al (2005) Colitis in mice lacking the common cytokine receptor γ chain is mediated by IL-6-producing CD4T cells. Gastroenterology 128(4):922–934
Yu M et al (2018) Aryl Hydrocarbon receptor activation modulates intestinal epithelial barrier function by maintaining tight Junction Integrity. Int J Biol Sci 14(1):69–77
Günzel D, Fromm M (2012) Claudins and other tight Junction proteins. Compr Physiol 2(3):1819–1852
Oshima T, Miwa H (2016) Gastrointestinal mucosal barrier function and diseases. J Gastroenterol 51(8):768–778
Jiang N et al (2020) Andrographolide derivative AL-1 reduces intestinal permeability in dextran sulfate sodium (DSS)-induced mice colitis model. Life Sci, 241
Vendrig JC, Fink-Gremmels J (2012) Intestinal barrier function in neonatal foals: options for improvement. Vet J 193(1):32–37
Mohamed SS et al (2021) Effect of the standard herbal preparation, STW5, treatment on dysbiosis induced by dextran sodium sulfate in experimental colitis. Bmc Complement Med Ther, 21(1)
Zou LJ et al (2019) Identification of microRNA transcriptome reveals that miR-100 is involved in the renewal of porcine intestinal epithelial cells. Sci China-Life Sci 62(6):816–828
Zhang F et al (2019) The Impact of on the Gut Microbiota of Mice with DSS-Induced Colitis Biomed Research International, 2019
Shang LJ et al (2021) Core altered microorganisms in Colitis Mouse Model: a Comprehensive Time-Point and Fecal Microbiota Transplantation Analysis. Antibiotics-Basel, 10(6)
Seo B et al (2024) Strain-specific anti-inflammatory effects of strain KBL1027 in koreans. Probiotics and Antimicrobial Proteins
Zang ZZ et al (2024) Study on the ameliorative effect of honeysuckle on DSS-induced ulcerative colitis in mice. J Ethnopharmacol, 325
Funding
This work was supported by the National Key Research and Development Program of China (No.2023YFD1801000) and the Interdisciplinary Integration and Innovation Project of JLU (No. JLUXKJC2021ZY08).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All experimental procedures were performed according to the guidelines of Animal Investigation Ethics Committee in Jilin University (No. SY202010004). They were therefore performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Competing interest
No competing financial interests exist.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yu, F., Wang, X., Ren, H. et al. Lactobacillus paracasei Jlus66 relieves DSS-induced ulcerative colitis in a murine model by maintaining intestinal barrier integrity, inhibiting inflammation, and improving intestinal microbiota structure. Eur J Nutr (2024). https://doi.org/10.1007/s00394-024-03419-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00394-024-03419-6