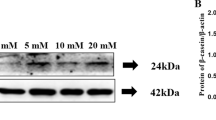
Abstract
Background and aims
Amino acids (AAs) not only constitute milk protein but also stimulate milk synthesis through the activation of mTORC1 signaling, but which amino acids that have the greatest impact on milk fat and protein synthesis is still very limited. In this study, we aimed to identify the most critical AAs involved in the regulation of milk synthesis and clarify how these AAs regulate milk synthesis through the G-protein-coupled receptors (GPCRs) signaling pathway.
Methods
In this study, a mouse mammary epithelial cell line (HC11) and porcine mammary epithelial cells (PMECs) were selected as study subjects. After treatment with different AAs, the amount of milk protein and milk fat synthesis were detected. Activation of mTORC1 and GPCRs signaling induced by AAs was also investigated.
Results
In this study, we demonstrate that essential amino acids (EAAs) are crucial to promote lactation by increasing the expression of genes and proteins related to milk synthesis, such as ACACA, FABP4, DGAT1, SREBP1, α-casein, β-casein, and WAP in HC11 cells and PMECs. In addition to activating mTORC1, EAAs uniquely regulate the expression of calcium-sensing receptor (CaSR) among all amino-acid-responsive GPCRs, which indicates a potential link between CaSR and the mTORC1 pathway in mammary gland epithelial cells. Compared with other EAAs, leucine and arginine had the greatest capacity to trigger GPCRs (p-ERK) and mTORC1 (p-S6K1) signaling in HC11 cells. In addition, CaSR and its downstream G proteins Gi, Gq, and Gβγ are involved in the regulation of leucine- and arginine-induced milk synthesis and mTORC1 activation. Taken together, our data suggest that leucine and arginine can efficiently trigger milk synthesis through the CaSR/Gi/mTORC1 and CaSR/Gq/mTORC1 pathways.
Conclusion
We found that the G-protein-coupled receptor CaSR is an important amino acid sensor in mammary epithelial cells. Leucine and arginine promote milk synthesis partially through the CaSR/Gi/mTORC1 and CaSR/Gq/mTORC1 signaling systems in mammary gland epithelial cells. Although this mechanism needs further verification, it is foreseeable that this mechanism may provide new insights into the regulation of milk synthesis.









Similar content being viewed by others
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Kim SW, Wu G (2009) Regulatory role for amino acids in mammary gland growth and milk synthesis. Amino Acids 37(1):89–95
Wu G, Bazer FW, Dai Z, Li D, Wang J, Wu Z (2014) Amino acid nutrition in animals: protein synthesis and beyond. Annu Rev Anim Biosci 2(1):387–417
Zhang S, Chen F, Zhang Y, Lv Y, Heng J, Min T, Li L, Guan W (2018) Recent progress of porcine milk components and mammary gland function. J Anim Sci Biotechnol 9(1):1–13
Lei J, Feng D, Zhang Y, Zhao F-Q, Wu Z, San Gabriel A, Fujishima Y, Uneyama H, Wu G (2012) Nutritional and regulatory role of branched-chain amino acids in lactation. Front Biosci 17(725):722
Manjarin R, Bequette BJ, Wu G, Trottier NL (2014) Linking our understanding of mammary gland metabolism to amino acid nutrition. Amino Acids 46(11):2447–2462
Lei J, Feng D, Zhang Y, Dahanayaka S, Li X, Yao K, Wang J, Wu Z, Dai Z, Wu G (2012) Regulation of leucine catabolism by metabolic fuels in mammary epithelial cells. Amino Acids 43(5):2179–2189
Zhang S, Zeng X, Ren M, Mao X, Qiao S (2017) Novel metabolic and physiological functions of branched chain amino acids: a review. J Anim Sci Biotechnol 8(1):1–12
Zhang S, Heng J, Song H, Zhang Y, Lin X, Tian M, Chen F, Guan W (2019) Role of maternal dietary protein and amino acids on fetal programming, early neonatal development, and lactation in swine. Animals 9(1):19
Ge Y, Li F, He Y, Cao Y, Guo W, Hu G, Liu J, Fu SJJoAP, Nutrition A (2022) L‐arginine stimulates the proliferation of mouse mammary epithelial cells and the development of mammary gland in pubertal mice by activating the GPRC6A/PI3K/AKT/mTOR signalling pathway. 106(6):1383–1395
Kim SW, Wu GJAa (2009) Regulatory role for amino acids in mammary gland growth and milk synthesis. 37:89–95
Appuhamy JRN, Knoebel NA, Nayananjalie WD, Escobar J, Hanigan MDJTJon (2012) Isoleucine and leucine independently regulate mTOR signaling and protein synthesis in MAC-T cells and bovine mammary tissue slices. 142(3):484–491
Lei J, Feng D, Zhang Y, Zhao F-Q, Wu Z, San Gabriel A, Fujishima Y, Uneyama H, Wu GJFiB-L (2012) Nutritional and regulatory role of branched-chain amino acids in lactation. 17 (7):2725–2739
Innis SM (2011) Dietary triacylglycerol structure and its role in infant nutrition. Adv Nutr 2(3):275–283
Wu Z, Tian M, Heng J, Chen J, Chen F, Guan W, Zhang S (2020) Current evidences and future perspectives for AMPK in the regulation of milk production and mammary gland biology. Front Cell Dev Biol 8:530
Abu-Elheiga L, Brinkley WR, Zhong L, Chirala SS, Woldegiorgis G, Wakil SJ (2000) The subcellular localization of acetyl-CoA carboxylase 2. Proc Natl Acad Sci 97(4):1444–1449
Li N, Zhao F, Wei C, Liang M, Zhang N, Wang C, Li Q-Z, Gao X-J (2014) Function of SREBP1 in the milk fat synthesis of dairy cow mammary epithelial cells. Int J Mol Sci 15(9):16998–17013
Che L, Xu M, Gao K, Zhu C, Wang L, Yang X, Wen X, Xiao H, Jiang Z, Wu D (2019) Valine increases milk fat synthesis in mammary gland of gilts through stimulating AKT/MTOR/SREBP1 pathway. Biol Reprod 101(1):126–137
Yang D, Jiang T, Liu J, Hong J, Lin P, Chen H, Zhou D, Tang K, Wang A, Jin Y (2018) Hormone regulates endometrial function via cooperation of endoplasmic reticulum stress and mTOR-autophagy. J Cell Physiol 233(9):6644–6659
Burgos S, Kim J, Dai M, Cant J (2013) Energy depletion of bovine mammary epithelial cells activates AMPK and suppresses protein synthesis through inhibition of mTORC1 signaling. Horm Metab Res 45(03):183–189
Jiang W, Zhu Z, Thompson HJ (2008) Dietary energy restriction modulates the activity of AMP-activated protein kinase, Akt, and mammalian target of rapamycin in mammary carcinomas, mammary gland, and liver. Can Res 68(13):5492–5499
Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DMJC (2010) Ragulator-rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. 141(2):290–303
Morton R (1962) Biochemistry and nutrition. Nature 193(4810):15–17
Dong X, Zhou Z, Saremi B, Helmbrecht A, Wang Z, Loor J (2018) Varying the ratio of Lys: Met while maintaining the ratios of Thr: Phe, Lys: Thr, Lys: His, and Lys: Val alters mammary cellular metabolites, mammalian target of rapamycin signaling, and gene transcription. J Dairy Sci 101(2):1708–1718
Gao H, Zhao S, Zheng N, Zhang Y, Wang S, Zhou X, Wang J (2017) Combination of histidine, lysine, methionine, and leucine promotes β-casein synthesis via the mechanistic target of rapamycin signaling pathway in bovine mammary epithelial cells. J Dairy Sci 100(9):7696–7709
Li S, Hosseini A, Danes M, Jacometo C, Liu J, Loor JJ (2016) Essential amino acid ratios and mTOR affect lipogenic gene networks and miRNA expression in bovine mammary epithelial cells. J Anim Sci Biotechnol 7(1):1–11
Zhang Y, Wang P, Lin S, Mercier Y, Yin H, Song Y, Zhang X, Che L, Lin Y, Xu S (2018) mTORC1 signaling-associated protein synthesis in porcine mammary glands was regulated by the local available methionine depending on methionine sources. Amino Acids 50(1):105–115
Che L, Xu M, Gao K, Wang L, Yang X, Wen X, Xiao H, Jiang Z, Wu D (2019) Valine supplementation during late pregnancy in gilts increases colostral protein synthesis through stimulating mTOR signaling pathway in mammary cells. Amino Acids 51(10):1547–1559
Wu Z, Heng J, Tian M, Song H, Chen F, Guan W, Zhang S (2020) Amino acid transportation, sensing and signal transduction in the mammary gland: key molecular signalling pathways in the regulation of milk synthesis. Nutr Res Rev 33(2):287–297
Zheng L, Zhang W, Zhou Y, Li F, Wei H, Peng JJIJoMS (2016) Recent advances in understanding amino acid sensing mechanisms that regulate mTORC1. 17(10):1636
Shigemura N, Shirosaki S, Sanematsu K, Yoshida R, Ninomiya Y (2009) Genetic and molecular basis of individual differences in human umami taste perception. PLoS One 4(8):e6717
Wang J, Hua T, Liu Z-J (2020) Structural features of activated GPCR signaling complexes. Curr Opin Struct Biol 63:82–89
Jain R, Watson U, Vasudevan L, Saini DK (2018) ERK activation pathways downstream of GPCRs. Int Rev Cell Mol Biol 338:79–109
Conigrave AD, Hampson DRJP, therapeutics (2010) Broad-spectrum amino acid-sensing class C G-protein coupled receptors: molecular mechanisms, physiological significance and options for drug development. 127(3):252–260
Li X, Li P, Wang L, Zhang M, Gao X (2019) Lysine enhances the stimulation of fatty acids on milk fat synthesis via the GPRC6A-PI3K-FABP5 signaling in bovine mammary epithelial cells. J Agric Food Chem 67(25):7005–7015
Liu J, Wang Y, Li D, Wang Y, Li M, Chen C, Fang X, Chen H, Zhang C (2017) Milk protein synthesis is regulated by T1R1/T1R3, a G protein-coupled taste receptor, through the mTOR pathway in the mouse mammary gland. Mol Nutr Food Res 61(9):1601017
Wang Y, Liu J, Wu H, Fang X, Chen H, Zhang C (2017) Amino acids regulate mTOR pathway and milk protein synthesis in a mouse mammary epithelial cell line is partly mediated by T1R1/T1R3. Eur J Nutr 56(8):2467–2474
Ma Q, Hu S, Bannai M, Wu G (2018) l-Arginine regulates protein turnover in porcine mammary epithelial cells to enhance milk protein synthesis. Amino Acids 50(5):621–628
Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3(7):1–12
Wang F, Shi H, Wang S, Wang Y, Cao Z, Li S (2019) Amino acid metabolism in dairy cows and their regulation in milk synthesis. Curr Drug Metab 20(1):36–45
Wu G (2017) Principles of animal nutrition. CRC Press
Brown EM, MacLeod RJ (2001) Extracellular calcium sensing and extracellular calcium signaling. Physiol Rev 81(1):239–297
Mamillapalli R, VanHouten J, Dann P, Bikle D, Chang W, Brown E, Wysolmerski J (2013) Mammary-specific ablation of the calcium-sensing receptor during lactation alters maternal calcium metabolism, milk calcium transport, and neonatal calcium accrual. Endocrinology 154(9):3031
Laporta J, Keil KP, Vezina CM, Hernandez LL (2014) Peripheral serotonin regulates maternal calcium trafficking in mammary epithelial cells during lactation in mice. PLoS One 9(10):e110190
Kong X, Tan B, Yin Y, Gao H, Li X, Jaeger LA, Bazer FW, Wu G (2012) l-Arginine stimulates the mTOR signaling pathway and protein synthesis in porcine trophectoderm cells. J Nutr Biochem 23(9):1178–1183
Hu L, Chen Y, Cortes IM, Coleman DN, Dai H, Liang Y, Parys C, Fernandez C, Wang M, Loor JJ (2020) Supply of methionine and arginine alters phosphorylation of mechanistic target of rapamycin (mTOR), circadian clock proteins, and α-s1-casein abundance in bovine mammary epithelial cells. Food Funct 11(1):883–894
Gao H-n, Hu H, Zheng N, Wang J-q (2015) Leucine and histidine independently regulate milk protein synthesis in bovine mammary epithelial cells via mTOR signaling pathway. J Zhejiang Univ Sci B 16(6):560–572
Soliman GA (2013) The role of mechanistic target of rapamycin (mTOR) complexes signaling in the immune responses. Nutrients 5(6):2231–2257
Saxton RA, Knockenhauer KE, Wolfson RL, Chantranupong L, Pacold ME, Wang T, Schwartz TU, Sabatini DM (2016) Structural basis for leucine sensing by the Sestrin2-mTORC1 pathway. Science 351(6268):53–58
Appuhamy JRN, Knoebel NA, Nayananjalie WD, Escobar J, Hanigan MD (2012) Isoleucine and leucine independently regulate mTOR signaling and protein synthesis in MAC-T cells and bovine mammary tissue slices. J Nutr 142(3):484–491
Maxin G, Glasser F, Hurtaud C, Peyraud J-L, Rulquin H (2011) Combined effects of trans-10, cis-12 conjugated linoleic acid, propionate, and acetate on milk fat yield and composition in dairy cows. J Dairy Sci 94(4):2051–2059
Nafikov R, Schoonmaker J, Korn K, Noack K, Garrick D, Koehler K, Minick-Bormann J, Reecy J, Spurlock D, Beitz D (2013) Association of polymorphisms in solute carrier family 27, isoform A6 (SLC27A6) and fatty acid-binding protein-3 and fatty acid-binding protein-4 (FABP3 and FABP4) with fatty acid composition of bovine milk. J Dairy Sci 96(9):6007–6021
Schennink A, Heck JM, Bovenhuis H, Visker MH, van Valenberg HJ, van Arendonk JA (2008) Milk fatty acid unsaturation: genetic parameters and effects of stearoyl-CoA desaturase (SCD1) and acyl CoA: diacylglycerol acyltransferase 1 (DGAT1). J Dairy Sci 91(5):2135–2143
Qi H, Meng C, Jin X, Li X, Li P, Gao X (2018) Methionine promotes milk protein and fat synthesis and cell proliferation via the SNAT2-PI3K signaling pathway in bovine mammary epithelial cells. J Agric Food Chem 66(42):11027–11033
Qiu Y, Qu B, Zhen Z, Yuan X, Zhang L, Zhang M (2019) Leucine promotes milk synthesis in bovine mammary epithelial cells via the PI3K-DDX59 signaling. J Agric Food Chem 67(32):8884–8895
Li P, Zhou C, Li X, Yu M, Li M, Gao X (2019) CRTC2 is a key mediator of amino acid-induced milk fat synthesis in mammary epithelial cells. J Agric Food Chem 67(37):10513–10520
Velázquez-Villegas LA, López-Barradas AM, Torres N, Hernández-Pando R, León-Contreras JC, Granados O, Ortíz V, Tovar AR (2015) Prolactin and the dietary protein/carbohydrate ratio regulate the expression of SNAT2 amino acid transporter in the mammary gland during lactation. Biochimica et Biophysica Acta (BBA)-Biomembranes 1848(5):1157–1164
Zhou J, Jiang M, Shi Y, Song S, Hou X, Lin Y (2020) Prolactin regulates LAT1 expression via STAT5 (signal transducer and activator of transcription 5) signaling in mammary epithelial cells of dairy cows. J Dairy Sci 103(7):6627–6634
Pauloin A, Chanat E (2012) Prolactin and epidermal growth factor stimulate adipophilin synthesis in HC11 mouse mammary epithelial cells via the PI3-kinase/Akt/mTOR pathway. Biochimica et Biophysica Acta (BBA)-Mol Cell Res 1823(5):987–996
Mun H-C, Franks AH, Culverston EL, Krapcho K, Nemeth EF, Conigrave AD (2004) The Venus fly trap domain of the extracellular Ca2+-sensing receptor is required for l-amino acid sensing. J Biol Chem 279(50):51739–51744
Mine Y, Zhang H (2015) Calcium-sensing receptor (CaSR)-mediated anti-inflammatory effects of l-amino acids in intestinal epithelial cells. J Agric Food Chem 63(45):9987–9995
Mun H-c, Leach KM, Conigrave AD (2019) l-amino acids promote calcitonin release via a calcium-sensing receptor: Gq/11-mediated pathway in human C-cells. Endocrinology 160(7):1590–1599
Wang Y, Chandra R, Samsa LA, Gooch B, Fee BE, Cook JM, Vigna SR, Grant AO, Liddle RA (2011) Amino acids stimulate cholecystokinin release through the Ca2+-sensing receptor. Am J Physiol Gastroint Liver Physiol 300(4):G528-G537
VanHouten J, Dann P, McGeoch G, Brown EM, Krapcho K, Neville M, Wysolmerski JJ (2004) The calcium-sensing receptor regulates mammary gland parathyroid hormone–related protein production and calcium transport. J Clin Investig 113(4):598–608
VanHouten JN, Neville MC, Wysolmerski JJ (2007) The calcium-sensing receptor regulates plasma membrane calcium adenosine triphosphatase isoform 2 activity in mammary epithelial cells: a mechanism for calcium-regulated calcium transport into milk. Endocrinology 148(12):5943–5954
Di Mise A, Tamma G, Ranieri M, Centrone M, van den Heuvel L, Mekahli D, Levtchenko EN, Valenti G (2018) Activation of calcium-sensing receptor increases intracellular calcium and decreases cAMP and mTOR in PKD1 deficient cells. Sci Rep 8(1):1–14
Hernández-Bedolla MA, González-Domínguez E, Zavala-Barrera C, Gutiérrez-López TY, Hidalgo-Moyle JJ, Vázquez-Prado J, Sánchez-Torres C, Reyes-Cruz G (2016) Calcium-sensing-receptor (CaSR) controls IL-6 secretion in metastatic breast cancer MDA-MB-231 cells by a dual mechanism revealed by agonist and inverse-agonist modulators. Mol Cell Endocrinol 436:159–168
Hernández-Bedolla MA, Carretero-Ortega J, Valadez-Sánchez M, Vázquez-Prado J, Reyes-Cruz G (2015) Chemotactic and proangiogenic role of calcium sensing receptor is linked to secretion of multiple cytokines and growth factors in breast cancer MDA-MB-231 cells. Biochimica et Biophysica Acta (BBA)-Mol Cell Res 1853(1):166–182
Jin L, Qian Y, Zhou J, Dai L, Cao C, Zhu C, Li S (2019) Activated CRH receptors inhibit autophagy by repressing conversion of LC3BI to LC3BII. Cell Signal 58:119–130
Gong Y, Zhang D-D, Tang Z, Coate K, Siv W, Yin L, Covington B, Patel R, Yang B, Yang L (2021) Hyperaminoacidemia induces pancreatic α cell proliferation via synergism between mTORC1 and CaSR-Gq signaling pathways
Wang Q, Chen J, Zhang M, Wang H, Zeng Y, Huang Y, Xu C (2022) Autophagy induced by muscarinic acetylcholine receptor 1 mediates migration and invasion targeting Atg5 via AMPK/mTOR pathway in prostate cancer. J Oncol 2022
Gulati P, Gaspers LD, Dann SG, Joaquin M, Nobukuni T, Natt F, Kozma SC, Thomas AP, Thomas G (2008) Amino acids activate mTOR complex 1 via Ca2+/CaM signaling to hVps34. Cell Metab 7(5):456–465
Decuypere J-P, Kindt D, Luyten T, Welkenhuyzen K, Missiaen L, De Smedt H, Bultynck G, Parys JB (2013) mTOR-controlled autophagy requires intracellular Ca2+ signaling. PLoS One 8(4):e61020
Conigrave AD, Ward DT (2013) Calcium-sensing receptor (CaSR): pharmacological properties and signaling pathways. Best Pract Res Clin Endocrinol Metab 27(3):315–331
Alhosaini K, Azhar A, Alonazi A, Al-Zoghaibi F (2021) GPCRs: the most promiscuous druggable receptor of the mankind. Saudi Pharmaceut J 29(6):539–551
Lizarzaburu M, Turcotte S, Du X, Duquette J, Fu A, Houze J, Li L, Liu J, Murakoshi M, Oda K (2012) Discovery and optimization of a novel series of GPR142 agonists for the treatment of type 2 diabetes mellitus. Bioorg Med Chem Lett 22(18):5942–5947
Wang T, Li Z, Cvijic ME, Zhang L, Sum CS (2017) Measurement of cAMP for Gαs-and Gαi protein-coupled receptors (GPCRs).
Xie J, Ponuwei GA, Moore CE, Willars GB, Tee AR, Herbert TP (2011) cAMP inhibits mammalian target of rapamycin complex-1 and-2 (mTORC1 and 2) by promoting complex dissociation and inhibiting mTOR kinase activity. Cell Signal 23(12):1927–1935
Zhang S, Wang H, Melick CH, Jeong M-H, Curukovic A, Tiwary S, Lama-Sherpa TD, Meng D, Servage KA, James NG (2021) AKAP13 couples GPCR signaling to mTORC1 inhibition. PLoS Genet 17(10):e1009832
Funding
The research was funded by the following funds: National Key R&D Program of China (No. 2021YFD1300700), the Guangdong Basic and Applied Basic Research Foundation (No. 2021A1515010440 and 2023A1515012098), the Science and Technology Program of Guangzhou (No. 202102020056), the National Natural Science Foundation of China (No. 31802067 and 31872364), and the Anhui Province Key Laboratory of Animal Nutrition Regulation and Health (No. APKLANRH202105).
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Q., Chen, J., Liu, J. et al. Leucine and arginine enhance milk fat and milk protein synthesis via the CaSR/Gi/mTORC1 and CaSR/Gq/mTORC1 pathways. Eur J Nutr 62, 2873–2890 (2023). https://doi.org/10.1007/s00394-023-03197-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-023-03197-7