Abstract
Purpose
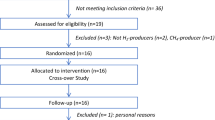
The aim of this pilot study was to analyze concomitantly the kinetics of production of 13C-labeled gut-derived metabolites from 13C-labeled wheat bran in three biological matrices (breath, plasma, stools), in order to assess differential fermentation profiles among subjects.
Methods
Six healthy women consumed a controlled breakfast containing 13C-labeled wheat bran biscuits. H2, CH4 and 13CO2, 13CH4 24 h-concentrations in breath were measured, respectively, by gas chromatography (GC) and GC-isotope ratio mass spectrometry (GC-IRMS). Plasma and fecal concentrations of 13C-short-chain fatty acids (linear SCFAs: acetate, propionate, butyrate, valerate; branched SCFAs: isobutyrate, isovalerate) were quantified using GC-combustion-IRMS. Gut microbiota composition was assessed by16S rRNA gene sequencing analysis.
Results
H2 and CH4 24 h-kinetics distinguished two groups in terms of fermentation-related gas excretion: high-CH4 producers vs low-CH4 producers (fasting concentrations: 45.3 ± 13.6 ppm vs 6.5 ± 3.6 ppm). Expired 13CH4 was enhanced and prolonged in high-CH4 producers compared to low-CH4 producers. The proportion of plasma and stool 13C-butyrate tended to be higher in low-CH4 producers, and inversely for 13C-acetate. Plasma branched SCFAs revealed different kinetics of apparition compared to linear SCFAs.
Conclusion
This pilot study allowed to consider novel procedures for the development of biomarkers revealing dietary fiber-gut microbiota interactions. The non-invasive assessment of exhaled gas following 13C-labeled fibers ingestion enabled to decipher distinct fermentation profiles: high-CH4 producers vs low-CH4 producers. The isotope labeling permits a specific in vivo characterisation of the dietary fiber impact consumption on microbiota metabolite production.
Clinical trial registration
The study has been registered under the number NCT03717311 at ClinicalTrials.gov on October 24, 2018.







Similar content being viewed by others
Data availability
Data will be available from the corresponding author on reasonable request.
References
Stephen AM, Champ MM-J, Cloran SJ, Fleith M, van Lieshout L, Mejborn H, Burley VJ (2017) Dietary fibre in Europe: current state of knowledge on definitions, sources, recommendations, intakes and relationships to health. Nutr Res Rev 30:149–190. https://doi.org/10.1017/S095442241700004X
Rezende ESV, Lima GC, Naves MMV (2021) Dietary fibers as beneficial microbiota modulators: A proposed classification by prebiotic categories. Nutr Burbank Los Angel Cty Calif. https://doi.org/10.1016/j.nut.2021.111217
Neyrinck AM, Rodriguez J, Vinoy S, Maquet V, Walter J, Bischoff SC, Laville M, Delzenne NM (2020) The FiberTAG project: Tagging dietary fibre intake by measuring biomarkers related to the gut microbiota and their interest for health. Nutr Bull 45:59–65. https://doi.org/10.1111/nbu.12416
Cronin P, Joyce SA, O’Toole PW, O’Connor EM (2021) Dietary fibre modulates the gut microbiota. Nutrients. https://doi.org/10.3390/nu13051655
Levitt MD (1969) Production and excretion of hydrogen gas in man. N Engl J Med 281:122–127. https://doi.org/10.1056/NEJM196907172810303
Smith NW, Shorten PR, Altermann EH, Roy NC, McNabb WC (2019) Hydrogen cross-feeders of the human gastrointestinal tract. Gut Microbes 10:270–288. https://doi.org/10.1080/19490976.2018.1546522
de Lacy Costello BPJ, Ledochowski M, Ratcliffe NM (2013) The importance of methane breath testing: a review. J Breath Res. https://doi.org/10.1088/1752-7155/7/2/024001
Mathur R, Kim G, Morales W, Sung J, Rooks E, Pokkunuri V, Weitsman S, Barlow GM, Chang C, Pimentel M (2013) Intestinal methanobrevibacter smithii but not total bacteria is related to diet-induced weight gain in rats. Obes Silver Spring Md 21:748–754. https://doi.org/10.1002/oby.20277
Mathur R, Amichai M, Chua KS, Mirocha J, Barlow GM, Pimentel M (2013) Methane and hydrogen positivity on breath test is associated with greater body mass index and body fat. J Clin Endocrinol Metab. https://doi.org/10.1210/jc.2012-3144
Armougom F, Henry M, Vialettes B, Raccah D, Raoult D (2009) Monitoring bacterial community of human gut microbiota reveals an increase in lactobacillus in obese patients and methanogens in anorexic patients. PLoS ONE. https://doi.org/10.1371/journal.pone.0007125
Boets E, Deroover L, Houben E, Vermeulen K, Gomand SV, Delcour JA, Verbeke K (2015) Quantification of in vivo colonic short chain fatty acid production from inulin. Nutrients 7:8916–8929. https://doi.org/10.3390/nu7115440
Boets E, Gomand SV, Deroover L, Preston T, Vermeulen K, De Preter V, Hamer HM, Van den Mooter G, De Vuyst L, Courtin CM, Annaert P, Delcour JA, Verbeke KA (2016) Systemic availability and metabolism of colonic-derived short-chain fatty acids in healthy subjects: a stable isotope study. J Physiol. https://doi.org/10.1113/JP272613
Blaak EE, Canfora EE, Theis S, Frost G, Groen AK, Mithieux G, Nauta A, Scott K, Stahl B, van Harsselaar J, van Tol R, Vaughan EE, Verbeke K (2020) Short chain fatty acids in human gut and metabolic health. Benef Microbes 11:411–455. https://doi.org/10.3920/BM2020.0057
Deroover L, Verspreet J, Luypaerts A, Vandermeulen G, Courtin CM, Verbeke K (2017) Wheat bran does not affect postprandial plasma Short-chain fatty acids from (13)C-inulin fermentation in healthy Subjects. Nutrients. https://doi.org/10.3390/nu9010083
Deroover L, Vázquez-Castellanos JF, Vandermeulen G, Luypaerts A, Raes J, Courtin CM, Verbeke K (2021) Wheat bran with reduced particle size increases serum SCFAs in obese subjects without improving health parameters compared with a maltodextrin placebo. Am J Clin Nutr. https://doi.org/10.1093/ajcn/nqab196
Verbeke K, Ferchaud-Roucher V, Preston T, Small AC, Henckaerts L, Krempf M, Wang H, Vonk RJ, Priebe MG (2010) Influence of the type of indigestible carbohydrate on plasma and urine short-chain fatty acid profiles in healthy human volunteers. Eur J Clin Nutr 64:678–684. https://doi.org/10.1038/ejcn.2010.92
van der Beek CM, Canfora EE, Kip AM, Gorissen SHM, Olde Damink SWM, van Eijk HM, Holst JJ, Blaak EE, Dejong CHC, Lenaerts K (2018) The prebiotic inulin improves substrate metabolism and promotes short-chain fatty acid production in overweight to obese men. Metabolism 87:25–35. https://doi.org/10.1016/j.metabol.2018.06.009
Péronnet F, Meynier A, Sauvinet V, Normand S, Bourdon E, Mignault D, St-Pierre DH, Laville M, Rabasa-Lhoret R, Vinoy S (2015) Plasma glucose kinetics and response of insulin and GIP following a cereal breakfast in female subjects: effect of starch digestibility. Eur J Clin Nutr 69:740–745. https://doi.org/10.1038/ejcn.2015.50
Ranaivo H, Zhang Z, Alligier M, Van Den Berghe L, Sothier M, Lambert-Porcheron S, Feugier N, Cuerq C, Machon C, Neyrinck AM, Seethaler B, Rodriguez J, Roumain M, Muccioli GG, Maquet V, Laville M, Bischoff SC, Walter J, Delzenne NM, Nazare J-A (2022) Chitin-glucan supplementation improved postprandial metabolism and altered gut microbiota in subjects at cardiometabolic risk in a randomized trial. Sci Rep 12:8830. https://doi.org/10.1038/s41598-022-12920-z
Costea PI, Zeller G, Sunagawa S, Pelletier E, Alberti A, Levenez F, Tramontano M, Driessen M, Hercog R, Jung FE, Kultima JR, Hayward MR, Coelho LP, Allen-Vercoe E, Bertrand L, Blaut M, Brown JRM, Carton T, Cools-Portier S, Daigneault M, Derrien M, Druesne A, Vos WMD, Finlay BB, Flint HJ, Guarner F, Hattori M, Heilig H, Luna RA, Vlieg JVH, Junick J, Klymiuk I, Langella P, Chatelier EL, Mai V, Manichanh C, Martin JC, Mery C, Morita H, O’Toole PW, Orvain C, Patil KR, Penders J, Persson S, Pons N, Popova M, Salonen A, Saulnier D, Scott KP, Singh B, Slezak K, Veiga P, Versalovic J, Zhao L, Zoetendal EG, Ehrlich SD, Dore J, Bork P (2017) Towards standards for human fecal sample processing in metagenomic studies. Nat Biotechnol 35:1069–1076. https://doi.org/10.1038/nbt.3960
Deehan EC, Yang C, Perez-Muñoz ME, Nguyen NK, Cheng CC, Triador L, Zhang Z, Bakal JA, Walter J (2020) Precision microbiome modulation with discrete dietary fiber structures directs short-chain fatty acid production. Cell Host Microbe 27:389-404.e6. https://doi.org/10.1016/j.chom.2020.01.006
Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP (2016) DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods 13:581–583. https://doi.org/10.1038/nmeth.3869
Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, Alexander H, Alm EJ, Arumugam M, Asnicar F, Bai Y, Bisanz JE, Bittinger K, Brejnrod A, Brislawn CJ, Brown CT, Callahan BJ, Caraballo-Rodríguez AM, Chase J, Cope EK, Da Silva R, Diener C, Dorrestein PC, Douglas GM, Durall DM, Duvallet C, Edwardson CF, Ernst M, Estaki M, Fouquier J, Gauglitz JM, Gibbons SM, Gibson DL, Gonzalez A, Gorlick K, Guo J, Hillmann B, Holmes S, Holste H, Huttenhower C, Huttley GA, Janssen S, Jarmusch AK, Jiang L, Kaehler BD, Kang KB, Keefe CR, Keim P, Kelley ST, Knights D, Koester I, Kosciolek T, Kreps J, Langille MGI, Lee J, Ley R, Liu Y-X, Loftfield E, Lozupone C, Maher M, Marotz C, Martin BD, McDonald D, McIver LJ, Melnik AV, Metcalf JL, Morgan SC, Morton JT, Naimey AT, Navas-Molina JA, Nothias LF, Orchanian SB, Pearson T, Peoples SL, Petras D, Preuss ML, Pruesse E, Rasmussen LB, Rivers A, Robeson MS, Rosenthal P, Segata N, Shaffer M, Shiffer A, Sinha R, Song SJ, Spear JR, Swafford AD, Thompson LR, Torres PJ, Trinh P, Tripathi A, Turnbaugh PJ, Ul-Hasan S, Van Der Hooft JJJ, Vargas F, Vázquez-Baeza Y, Vogtmann E, Von Hippel M, Walters W, Wan Y, Wang M, Warren J, Weber KC, Williamson CHD, Willis AD, Xu ZZ, Zaneveld JR, Zhang Y, Zhu Q, Knight R, Caporaso JG (2019) Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol 37:852–857. https://doi.org/10.1038/s41587-019-0209-9
Wang S, Tian W, Liu Y, Yan G, Fang S, Wang Y, Yu B (2021) Temporal trend of circulating trans-fatty acids and risk of long-term mortality in general population. Clin Nutr 40:1095–1101. https://doi.org/10.1016/j.clnu.2020.07.010
Morrison DJ, Cooper K, Waldron S, Slater C, Weaver LT, Preston T (2004) A streamlined approach to the analysis of volatile fatty acids and its application to the measurement of whole-body flux. Rapid Commun Mass Spectrom RCM 18:2593–2600. https://doi.org/10.1002/rcm.1662
Neyrinck AM, Rodriguez J, Zhang Z, Seethaler B, Mailleux F, Vercammen J, Bindels LB, Cani PD, Nazare J-A, Maquet V, Laville M, Bischoff SC, Walter J, Delzenne NM (2021) Noninvasive monitoring of fibre fermentation in healthy volunteers by analyzing breath volatile metabolites: lessons from the FiberTAG intervention study. Gut Microbes 13:1–16. https://doi.org/10.1080/19490976.2020.1862028
Conway de Macario E, Macario AJL (2009) Methanogenic archaea in health and disease: A novel paradigm of microbial pathogenesis. Int J Med Microbiol 299:99–108. https://doi.org/10.1016/j.ijmm.2008.06.011
Pochart P, Lémann F, Flourié B, Pellier P, Goderel I, Rambaud J-C (1993) Pyxigraphic sampling to enumerate methanogens and anaerobes in the right colon of healthy humans. Gastroenterology 105:1281–1285. https://doi.org/10.1016/0016-5085(93)90129-Z
Tannock GW, Lawley B, Munro K, Sims IM, Lee J, Butts CA, Roy N (2014) RNA-stable isotope probing (RNA-SIP) shows carbon utilization from inulin by specific bacterial populations in the large bowel of rats. Appl Environ Microbiol AEM. https://doi.org/10.1128/AEM.03799-13
Schink B (1997) Energetics of syntrophic cooperation in methanogenic degradation. Microbiol Mol Biol Rev MMBR 61:262–280. https://doi.org/10.1128/mmbr.61.2.262-280.1997
Roediger WE (1980) Role of anaerobic bacteria in the metabolic welfare of the colonic mucosa in man. Gut 21:793–798. https://doi.org/10.1136/gut.21.9.793
Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT (1987) Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 28:1221–1227. https://doi.org/10.1136/gut.28.10.1221
Rechkemmer G, Rönnau K, von Engelhardt W (1988) Fermentation of polysaccharides and absorption of short chain fatty acids in the mammalian hindgut. Comp Biochem Physiol A 90:563–568. https://doi.org/10.1016/0300-9629(88)90668-8
Bloemen JG, Olde Damink SWM, Venema K, Buurman WA, Jalan R, Dejong CHC (2010) Short chain fatty acids exchange: is the cirrhotic, dysfunctional liver still able to clear them? Clin Nutr Edinb Scotl 29:365–369. https://doi.org/10.1016/j.clnu.2009.10.002
Vogt PPB, Wolever T (2004) L-Rhamnose increases serum propionate in humans. Am J Clin Nutr 80:89
Alves-Bezerra M, Cohen DE (2017) Triglyceride metabolism in the liver. Compr Physiol 8:1–8. https://doi.org/10.1002/cphy.c170012
Jakobsdottir G, Bjerregaard JH, Skovbjerg H, Nyman M (2013) Fasting serum concentration of short-chain fatty acids in subjects with microscopic colitis and celiac disease: no difference compared with controls, but between genders. Scand J Gastroenterol 48:696–701. https://doi.org/10.3109/00365521.2013.786128
Powers L, Osborn MK, Yang D, Kien CL, Murray RD, Beylot M, Brunengraber H (1995) Assay of the concentration and stable isotope enrichment of short-chain fatty acids by gas chromatography/mass spectrometry. J Mass Spectrom 30:747–754. https://doi.org/10.1002/jms.1190300514
Pouteau E, Meirim I, Métairon S, Fay LB (2001) Acetate, propionate and butyrate in plasma: determination of the concentration and isotopic enrichment by gas chromatography/mass spectrometry with positive chemical ionization. J Mass Spectrom JMS 36:798–805. https://doi.org/10.1002/jms.181
Rahman MN, Diantini A, Fattah M, Barliana MI, Wijaya A (2021) A highly sensitive, simple, and fast gas chromatography-mass spectrometry method for the quantification of serum short-chain fatty acids and their potential features in central obesity. Anal Bioanal Chem 413:6837–6844. https://doi.org/10.1007/s00216-021-03639-3
Zhao G, Nyman M, Jönsson JA (2006) Rapid determination of short-chain fatty acids in colonic contents and faeces of humans and rats by acidified water-extraction and direct-injection gas chromatography. Biomed Chromatogr BMC 20:674–682. https://doi.org/10.1002/bmc.580
Rodriguez J, Neyrinck AM, Zhang Z, Seethaler B, Nazare J-A, Robles Sánchez C, Roumain M, Muccioli GG, Bindels LB, Cani PD, Maquet V, Laville M, Bischoff SC, Walter J, Delzenne NM (2020) Metabolite profiling reveals the interaction of chitin-glucan with the gut microbiota. Gut Microbes 12:1810530. https://doi.org/10.1080/19490976.2020.1810530
Rios-Covian D, González S, Nogacka AM, Arboleya S, Salazar N, Gueimonde M, de los Reyes-Gavilán CG, (2020) An overview on fecal branched short-chain fatty acids along human life and as related with body mass index: associated dietary and anthropometric factors. Front Microbiol 11:973. https://doi.org/10.3389/fmicb.2020.00973
Macfarlane GT, Gibson GR, Cummings JH (1992) Comparison of fermentation reactions in different regions of the human colon. J Appl Bacteriol 72:57–64. https://doi.org/10.1111/j.1365-2672.1992.tb04882.x
Rios-Covian D, González S, Nogacka AM, Arboleya S, Salazar N, Gueimonde M, de los Reyes-Gavilán CG (2020) An Overview on Fecal Branched Short-Chain Fatty Acids Along Human Life and as Related With Body Mass Index: Associated Dietary and Anthropometric Factors. Front Microbiol 11:
Le Gall G, Noor SO, Ridgway K, Scovell L, Jamieson C, Johnson IT, Colquhoun IJ, Kemsley EK, Narbad A (2011) Metabolomics of fecal extracts detects altered metabolic activity of gut microbiota in ulcerative colitis and irritable bowel syndrome. J Proteome Res 10:4208–4218. https://doi.org/10.1021/pr2003598
Heimann E, Nyman M, Pålbrink A-K, Lindkvist-Petersson K, Degerman E (2016) Branched short-chain fatty acids modulate glucose and lipid metabolism in primary adipocytes. Adipocyte 5:359–368. https://doi.org/10.1080/21623945.2016.1252011
Ratajczak W, Mizerski A, Rył A, Słojewski M, Sipak O, Piasecka M, Laszczyńska M (2021) Alterations in fecal short chain fatty acids (SCFAs) and branched short-chain fatty acids (BCFAs) in men with benign prostatic hyperplasia (BPH) and metabolic syndrome (MetS). Aging. https://doi.org/10.18632/aging.202968
Granado-Serrano AB, Martín-Garí M, Sánchez V, Riart Solans M, Berdún R, Ludwig IA, Rubió L, Vilaprinyó E, Portero-Otín M, Serrano JCE (2019) Faecal bacterial and short-chain fatty acids signature in hypercholesterolemia. Sci Rep 9:1772. https://doi.org/10.1038/s41598-019-38874-3
Szczesniak O, Hestad KA, Hanssen JF, Rudi K (2016) Isovaleric acid in stool correlates with human depression. Nutr Neurosci 19:279–283. https://doi.org/10.1179/1476830515Y.0000000007
Velázquez M, Davies C, Marett R, Slavin JL, Feirtag JM (2000) Effect of Oligosaccharides and fibre substitutes on short-chain fatty acid production by human faecal microflora. Anaerobe 6:87–92. https://doi.org/10.1006/anae.1999.0318
Ranaivo H, Thirion F, Béra-Maillet C, Guilly S, Simon C, Sothier M, Van Den Berghe L, Feugier-Favier N, Lambert-Porcheron S, Dussous I, Roger L, Roume H, Galleron N, Pons N, Le Chatelier E, Ehrlich SD, Laville M, Doré J, Nazare J-A (2022) Increasing the diversity of dietary fibers in a daily-consumed bread modifies gut microbiota and metabolic profile in subjects at cardiometabolic risk. Gut Microbes 14:2044722. https://doi.org/10.1080/19490976.2022.2044722
Acknowledgements
We thank all the staff of the Centre de Recherche en Nutrition Humaine Rhône-Alpes and more particularly N. Feugier, S. Lambert-Porcheron, L. Van Den Berghe, M. Sothier, E. Brossa, A. Samperiz, C. Louche-Pélissier and A. Cestre for their excellent technical assistance in subject recruitment, sample collection and analysis, and dietary surveys. We thank the six subjects who participated in this study. 13C-Wheat bran biscuits were designed and made by the Nutrition Department of Mondelēz International, Saclay, France. 13CH4 measurements were performed by Sam Barker and Rob Berstan from Elementar UK Ltd (data presented). The Stable Isotope Facility at UC Davis performed the analysis of duplicate samples (identical results not shown). NMD is a recipient of grants from the Fonds de la Recherche Scientifique (FRS-FNRS) [PINT-MULTI R.8013.19 (NEURONERANET, call 2019) and PDR T.0068.19].
Funding
FiberTAG project was initiated from a European Joint Programming Initiative ‘A Healthy Diet for a Healthy Life’ (JPI HDHL). Funding for this article is provided by the Agence Nationale de la Recherche (ANR, France).
Author information
Authors and Affiliations
Contributions
LM, VS, AEB, AM, SCB, JW, AMN, ML, NMD, SV and JAN contributed to the study design. AEB contributed to data collection. LM, VS, CM and AM performed the analysis. LM, VS, HR and JAN wrote the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
A Meynier and S Vinoy are Mondelez International employees. No potential conflict of interest was reported by the other author(s).
Informed consent
The study protocol was in line with the Declaration of Helsinki and was approved by the Ethics Committee (Comité de Protection des Personnes Nord-Ouest IV, France, Registration Number: 2018 A00949 46). All participants signed written informed consent.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Meiller, L., Sauvinet, V., Breyton, AE. et al. Metabolic signature of 13C-labeled wheat bran consumption related to gut fermentation in humans: a pilot study. Eur J Nutr 62, 2633–2648 (2023). https://doi.org/10.1007/s00394-023-03161-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-023-03161-5