Abstract
Purpose
To study the effects of feeding docosahexaenoic acid (DHA, derived from novel canola oil), with same amount of arachidonic acid (ARA), supplemented diet to lactating dams on the immune system development of suckled offspring using a T helper type-2 (Th2)-dominant BALB/c mouse.
Methods
Dams received nutritionally complete control (no ARA or DHA) or DHA + ARA diet (1% DHA and 1% ARA of total fatty acids) from 5 days pre-parturition to the end of 3-week suckling period. After euthanization, relevant tissues were collected to study fatty acids, splenocyte phenotype and function (ex vivo cytokines with/without lipopolysaccharide (LPS, bacterial challenge) or phorbol myristate acetate + ionomycin (PMAi) stimulation).
Results
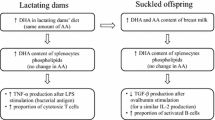
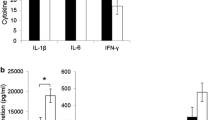
Feeding dams a DHA diet significantly increased the mammary gland milk phospholipid concentration of DHA and ARA. This resulted in 60% higher DHA levels in splenocyte phospholipids of the pups although ARA levels showed no difference. In dams fed DHA diet, significantly higher proportion of CD27+ cytotoxic T cell (CTL) and CXCR3+ CCR6- Th (enriched in Th1) were observed than control, but there were no differences in the splenocyte function upon PMAi (non-specific lymphocyte stimulant) stimulation. Pups from DHA-fed dams showed significantly higher IL-1β, IFN-γ and TNF-α (inflammatory cytokines) by LPS-stimulated splenocytes. This may be due to higher proportion of CD86+ macrophages and B cells (all p’s < 0.05) in these pups, which may influence T cell polarization.
Conclusion
Plant-based source of DHA in maternal diet resulted in higher ex vivo production of inflammatory cytokines by splenocytes due to change in their phenotype, and this can skew T cell towards Th1 response in a Th2-dominant BALB/c mouse.




Similar content being viewed by others
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Change history
17 October 2023
A Correction to this paper has been published: https://doi.org/10.1007/s00394-023-03264-z
Abbreviations
- DHA:
-
Docosahexaenoic acid
- ARA:
-
Arachidonic acid
- ALA:
-
Alpha-linolenic acid
- LA:
-
Linoleic acid
- Th2:
-
T helper type-2
- LCPUFA:
-
Long-chain polyunsaturated fatty acid
- CD:
-
Cluster of differentiation
- NK:
-
Natural killer
- TNF-α:
-
Tumour necrosis factor-alpha
- IFN-γ:
-
Interferon-gamma
- IL:
-
Interleukin
- CXCL1:
-
C-X-C motif ligand-1
- CTLA-4:
-
Cytotoxic T lymphocyte-associated protein 4
References
Saavedra JM, Dattilo AM (2017) Nutrition in the first 1000 days of life: society’s greatest opportunity. Early nutrition and long-term health. Elsevier. https://doi.org/10.1016/b978-0-08-100168-4.00025-2
Calder PC, Krauss-Etschmann S, de Jong EC, Dupont C, Frick JS, Frokiaer H, Heinrich J, Garn H, Koletzko S, Lack G, Mattelio G, Renz H, Sangild PT, Schrezenmeir J, Stulnig TM, Thymann T, Wold AE, Koletzko B (2006) Early nutrition and immunity—progress and perspectives. Br J Nutr 96(4):774–790
Kelly D, Coutts AGP (2000) Early nutrition and the development of immune function in the neonate. Proc Nutr Soc 59(2):177–185. https://doi.org/10.1017/s0029665100000197
Basha S, Surendran N, Pichichero M (2014) Immune responses in neonates. Expert Rev Clin Immunol 10(9):1171–1184. https://doi.org/10.1586/1744666X.2014.942288
Pérez-Cano FJ, Castellote C, González-Castro AM, Pelegrí C, Castell M, Franch À (2005) Developmental changes in intraepithelial T lymphocytes and NK cells in the small intestine of neonatal rats. Pediatr Res 58:885–891. https://doi.org/10.1203/01.pdr.0000182187.88505.49
Pérez-Cano FJ, Castellote C, Marín-Gallén S, González-Castro A, Franch À, Castell M (2007) Phenotypic and functional characteristics of rat spleen lymphocytes during suckling. Dev Comp Immunol 31(12):1264–1277. https://doi.org/10.1016/j.dci.2007.03.004
Pérez-Cano FJ, Franch Á, Castellote C, Castell M (2012) The suckling rat as a model for immunonutrition studies in early life. Clin Dev Immunol. https://doi.org/10.1155/2012/537310
Drutman SB, Kendall JC, Trombetta ES (2012) Inflammatory spleen monocytes can upregulate CD11c expression without converting into dendritic cells. J Immunol 188(8):3603–3610. https://doi.org/10.4049/jimmunol.1102741
Prescott SL (2003) Early origins of allergic disease: a review of processes and influences during early immune development. Curr Opin Allergy Clin Immunol 3(2):125–132. https://doi.org/10.1097/01.all.0000064776.57552.32
Wilson CB, Kollmann TR (2008) Induction of antigen-specific immunity in human neonates and infants. Nestle Nutr Workshop Ser Pediatr 61:183–195. https://doi.org/10.1159/000113493
Zaghouani H, Hoeman CM, Adkins B (2009) Neonatal immunity: faulty T-helpers and the shortcomings of dendritic cells. Trends Immunol 30(12):585–591. https://doi.org/10.1016/j.it.2009.09.002
Raphael I, Nalawade S, Eagar TN, Forsthuber TG (2015) T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine 74(1):5–17. https://doi.org/10.1016/j.cyto.2014.09.011
Van Der Velden VHJ, Laan MP, Baert MRM, De Waal MR, Neijens HJ, Savelkoul HFJ (2001) Selective development of a strong Th2 cytokine profile in high-risk children who develop atopy: risk factors and regulatory role of IFN-γ, IL-4 and IL-10. Clin Exp Allergy 31(7):997–1006. https://doi.org/10.1046/j.1365-2222.2001.01176.x
Kim W, Khan NA, McMurray DN, Prior IA, Wang N, Chapkin RS (2010) Regulatory activity of polyunsaturated fatty acids in T cell signaling. Prog Lipid Res 49(3):250–261. https://doi.org/10.1016/j.plipres.2010.01.002
Calder PC (2008) The relationship between the fatty acid composition of immune cells and their function. Prostaglandins Leukot Essent Fatty Acids 79(3):101–108. https://doi.org/10.1016/j.plefa.2008.09.016
Field CJ, Clandinin MT, Van Aerde JE (2001) Polyunsaturated fatty acids and T cell function: implications for the neonate. Lipids 36(9):1025–1032. https://doi.org/10.1007/s11745-001-0813-6
Peterson LD, Jeffery NM, Thies F, Sanderson P, Newsholme EA, Calder PC (1998) Eicosapentaenoic and docosahexaenoic acids alter rat spleen leukocyte fatty acid composition and prostaglandin E 2 production but have different effects on lymphocyte functions and cell-mediated immunity. Lipids. https://doi.org/10.1007/s11745-998-0193-y
Clausen M, Jonasson K, Keil T, Beyer K, Sigurdardottir ST (2018) Fish oil in infancy protects against food allergy in Iceland—results from a birth cohort study. Allergy 73(6):1305–1312. https://doi.org/10.1111/all.13385
Nagakura T, Matsuda S, Shichijyo K, Sugimoto H, Hata K (2000) Dietary supplementation with fish oil rich in ω-3 polyunsaturated fatty acids in children with bronchial asthma. Eur Respir J 16(5):861–865. https://doi.org/10.1183/09031936.00.16586100
Kull I, Bergström A, Lilja G, Pershagen G, Wickman M (2006) Fish consumption during the first year of life and development of allergic diseases during childhood. Allergy 61(8):1009–1015. https://doi.org/10.1111/j.1398-9995.2006.01115.x
Alm B, Aberg N, Erdes L, Mollborg P, Pettersson R, Norvenius SG, Goksor E, Wennergren G (2009) Early introduction of fish decreases the risk of eczema in infants. Arch Dis Child 94(1):11–15. https://doi.org/10.1136/adc.2008.140418
Furuhjelm C, Jenmalm MC, Fälth-Magnusson K, Duchén K (2011) Th1 and Th2 chemokines, vaccine-induced immunity, and allergic disease in infants after maternal ω-3 fatty acid supplementation during pregnancy and lactation. Pediatr Res 69(3):259–264. https://doi.org/10.1203/pdr.0b013e3182072229
Richard C, Lewis ED, Field CJ (2016) Evidence for the essentiality of arachidonic and docosahexaenoic acid in the postnatal maternal and infant diet for the development of the infant’s immune system early in life. Appl Physiol Nutr Metab 41(5):461–475. https://doi.org/10.1139/apnm-2015-0660
van Goor SA, Dijck-Brouwer DAJ, Hadders-Algra M, Doornbos B, Erwich JJHM, Schaafsma A, Muskiet FAJ (2009) Human milk arachidonic acid and docosahexaenoic acid contents increase following supplementation during pregnancy and lactation. Prostaglandins Leukot Essent Fatty Acids 80(1):65–69. https://doi.org/10.1016/j.plefa.2008.11.002
Stables MJ, Gilroy DW (2011) Old and new generation lipid mediators in acute inflammation and resolution. Prog Lipid Res 50(1):35–51. https://doi.org/10.1016/j.plipres.2010.07.005
Calder PC (2013) Long chain fatty acids and gene expression in inflammation and immunity. Curr Opin Clin Nutr Metab Care 16:425–433. https://doi.org/10.1097/MCO.0b013e3283620616
Calder PC, Kremmyda L-S, Vlachava M, Noakes PS, Miles EA (2010) Is there a role for fatty acids in early life programming of the immune system? Proc Nutr Soc 69(3):373–380. https://doi.org/10.1017/s0029665110001552
Richard C, Lewis ED, Goruk S, Field CJ (2016) The content of docosahexaenoic acid in the suckling and the weaning diet beneficially modulates the ability of immune cells to response to stimuli. J Nutr Biochem 35:22–29. https://doi.org/10.1016/j.jnutbio.2016.05.014
Richard C, Lewis ED, Goruk S, Field CJ (2016) A dietary supply of docosahexaenoic acid early in life is essential for immune development and the establishment of oral tolerance in female rat offspring. J Nutr 146:2398–2406. https://doi.org/10.3945/jn.116.237149
Richard C, Lewis ED, Goruk S, Field CJ (2016) The content of docosahexaenoic acid in the maternal diet differentially affects the immune response in lactating dams and suckled offspring. Eur J Nutr 55(7):2255–2264. https://doi.org/10.1007/s00394-015-1035-6
Weise C, Heunemann C, Loddenkemper C, Herz U, van Tol EAF, Worm M (2011) Dietary docosahexaenoic acid in combination with arachidonic acid ameliorates allergen-induced dermatitis in mice. Pediatr Allergy Immunol 22(5):497–504. https://doi.org/10.1111/j.1399-3038.2010.01133.x
Patel D, Newell M, Goruk S, Richard C, Field CJ (2021) Long chain polyunsaturated fatty acids docosahexaenoic acid and arachidonic acid supplementation in the suckling and the post-weaning diet influences the immune system development of T Helper type-2 bias brown norway rat offspring. Front Nutr. https://doi.org/10.3389/fnut.2021.769293
Patel D, Goruk S, Richard C, Field CJ (2022) Combined supplementation with arachidonic and docosahexaenoic acids in t helper type-2 skewed brown Norway rat offspring is beneficial in the induction of oral tolerance toward ovalbumin and immune system development. J Nutr 152(9):2165–2178. https://doi.org/10.1093/jn/nxac118
Brenna TJ, Varamini B, Jensen RG, Diersen-Schade DA, Boettcher JA, Arterburn LM (2007) Docosahexaenoic and arachidonic acid concentrations in human breast milk worldwide. Am J Clin Nutr 85:1457–1464
Standard for infant formula and formulas for special medical purposes intended for infants (2007). Codex Stan, vol 72. Codex Alimentarius Commission, Rome
Lewis ED, Richard C, Goruk S, Dellschaft NS, Curtis JM, Jacobs RL, Field CJ (2016) The form of choline in the maternal diet affects immune development in suckled rat offspring. J Nutr 146(4):823–830. https://doi.org/10.3945/jn.115.225888
Field CJ, Wu G, Métroz-Dayer MD, Montambault M, Marliss EB (1990) Lactate production is the major metabolic fate of glucose in splenocytes and is altered in spontaneously diabetic BB rats. Biochem J 272(2):445–452. https://doi.org/10.1042/bj2720445
Blewett HJ, Gerdung CA, Ruth MR, Proctor SD, Field CJ (2009) Vaccenic acid favourably alters immune function in obese JCR:LA-cp rats. Br J Nutr 102:526–536. https://doi.org/10.1017/S0007114509231722
Abbas AK (2020) The surprising story of IL-2. Am J Pathol 190(9):1776–1781. https://doi.org/10.1016/j.ajpath.2020.05.007
Field CJ, Thomson CA, Van Aerde JE, Parrott A, Art E, Lien E, Clandinin MT (2000) Lower proportion of CD45R0+ cells and deficient interleukin-10 production by formula-fed infants, compared with human-fed, is corrected with supplementation of long-chain polyunsaturated fatty acids. J Pediatr Gastroenterol Nutr 31:291–299. https://doi.org/10.1097/00005176-200009000-00017
Field CJ, Ryan EA, Thomson AB, Clandinin MT (1988) Dietary fat and the diabetic state alter insulin binding and the fatty acyl composition of the adipocyte plasma membrane. Biochem J 253(2):417–424. https://doi.org/10.1042/bj2530417
Cruz-Hernandez C, Deng Z, Zhou J, Hill AR, Yurawecz MP, Delmonte P, Mossoba MM, Dugan MER, Kramer JKG (2004) Methods for analysis of conjugated linoleic acids and trans-18:1 isomers in dairy fats by using a combination of gas chromatography, silver-ion thin-layer chromatography/gas chromatography, and silver-ion liquid chromatography. J AOAC Int 87:545–562
Mukherjee S, Maiti PK, Nandi D (2002) Role of CD80, CD86, and CTLA4 on mouse CD4 <sup>+</sup> T lymphocytes in enhancing cell-cycle progression and survival after activation with PMA and ionomycin. J Leukoc Biol 72(5):921–931. https://doi.org/10.1189/jlb.72.5.921
Borst J, Hendriks J, Xiao Y (2005) CD27 and CD70 in T cell and B cell activation. Curr Opin Immunol 17(3):275–281. https://doi.org/10.1016/j.coi.2005.04.004
Richard C, Lewis ED, Goruk S, Field CJ (2016) Feeding a diet enriched in docosahexaenoic acid to lactating dams improves the tolerance response to egg protein in suckled pups. Nutrients. https://doi.org/10.3390/nu8020103
D’Vaz N, Meldrum SJ, Dunstan JA, Lee-Pullen TF, Metcalfe J, Holt BJ, Serralha M, Tulic MK, Mori TA, Prescott SL (2012) Fish oil supplementation in early infancy modulates developing infant immune responses. Clin Exp Allergy 42(8):1206–1216. https://doi.org/10.1111/j.1365-2222.2012.04031.x
Lauritzen L, Kjaer TMR, Fruekilde M-B, Michaelsen KF, Frokiaer H (2005) Fish oil supplementation of lactating mothers affects cytokine production in 2 1/2-year-old children. Lipids 40(7):669–676. https://doi.org/10.1007/s11745-005-1429-6
Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM (2000) M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol 164(12):6166–6173. https://doi.org/10.4049/jimmunol.164.12.6166
Kuroda E, Sugiura T, Zeki K, Yoshida Y, Yamashita U (2000) Sensitivity difference to the suppressive effect of prostaglandin E<sub>2</sub>Among mouse strains: a possible mechanism to polarize Th2 type response in BALB/c mice. J Immunol 164(5):2386–2395. https://doi.org/10.4049/jimmunol.164.5.2386
Carlsson JA, Wold AE, Sandberg A-S, Östman SM (2015) The polyunsaturated fatty acids arachidonic acid and docosahexaenoic acid induce mouse dendritic cells maturation but reduce T cell responses in vitro. PLoS ONE 10(11):e0143741. https://doi.org/10.1371/journal.pone.0143741
Søyland E, Lea T, Sandstad B, Drevon A (1994) Dietary supplementation with very long-chain n-3 fatty acids in man decreases expression of the interleukin-2 receptor (CD25) on mitogen-stimulated lymphocytes from patients with inflammatory skin diseases. Eur J Clin Invest 24(4):236–242. https://doi.org/10.1111/j.1365-2362.1994.tb01080.x
Gutiérrez S, Svahn SL, Johansson ME (2019) Effects of omega-3 fatty acids on immune cells. Int J Mol Sci 20(20):5028
Zaloga GP (2021) Narrative review of n-3 polyunsaturated fatty acid supplementation upon immune functions, resolution molecules and lipid peroxidation. Nutrients 13(2):662. https://doi.org/10.3390/nu13020662
McMurray DN, Jolly CA, Chapkin RS (2000) Effects of dietary n-3 fatty acids on T cell activation and T cell receptor-mediated signaling in a murine model. J Infect Dis 182(s1):S103–S107. https://doi.org/10.1086/315909
Rockett BD, Teague H, Harris M, Melton M, Williams J, Wassall SR, Shaikh SR (2012) Fish oil increases raft size and membrane order of B cells accompanied by differential effects on function. J Lipid Res 53(4):674–685. https://doi.org/10.1194/jlr.m021782
Gurzell EA, Teague H, Harris M, Clinthorne J, Shaikh SR, Fenton JI (2013) DHA-enriched fish oil targets B cell lipid microdomains and enhances ex vivo and in vivo B cell function. J Leukoc Biol 93:463–470. https://doi.org/10.1189/jlb.0812394
Teague H, Harris M, Fenton J, Lallemand P, Shewchuk BM, Shaikh SR (2014) Eicosapentaenoic and docosahexaenoic acid ethyl esters differentially enhance B-cell activity in murine obesity. J Lipid Res 55(7):1420–1433
Martin MD, Badovinac VP (2018) Defining memory CD8 T cell. Front Immunol. https://doi.org/10.3389/fimmu.2018.02692
Hendriks J, Xiao Y, Borst J (2003) CD27 promotes survival of activated T cells and complements CD28 in generation and establishment of the effector T cell pool. J Exp Med 198(9):1369–1380. https://doi.org/10.1084/jem.20030916
Granot E, Jakobovich E, Rabinowitz R, Levy P, Schlesinger M (2011) DHA supplementation during pregnancy and lactation affects infants’ cellular but not humoral immune response. Mediat Inflamm 2011:493925. https://doi.org/10.1155/2011/493925
Myles IA, Datta SK (2021) Frontline science: breast milk confers passive cellular immunity via CD8-dependent mechanisms. J Leukoc Biol 109(4):709–715. https://doi.org/10.1002/JLB.3HI0820-406RR
Field CJ (2005) The immunological components of human milk and their effect on immune development in infants. J Nutr 135(1):1–4. https://doi.org/10.1093/jn/135.1.1
Duchamp M, Sterlin D, Diabate A, Uring-Lambert B, Guérin-El Khourouj V, Le Mauff B, Monnier D, Malcus C, Labalette M, Picard C (2014) B-cell subpopulations in children: national reference values. Immun Inflamm Dis 2(3):131–140. https://doi.org/10.1002/iid3.26
Tuaillon E, Valea D, Becquart P, Al Tabaa Y, Meda N, Bollore K, Van De Perre P, Vendrell J-P (2009) Human milk-derived B cells: a highly activated switched memory cell population primed to secrete antibodies. J Immunol 182(11):7155–7162. https://doi.org/10.4049/jimmunol.0803107
Hensel JA, Khattar V, Ashton R, Ponnazhagan S (2019) Characterization of immune cell subtypes in three commonly used mouse strains reveals gender and strain-specific variations. Lab Invest 99(1):93–106. https://doi.org/10.1038/s41374-018-0137-1
Klein SL, Flanagan KL (2016) Sex differences in immune responses. Nat Rev Immunol 16(10):626–638. https://doi.org/10.1038/nri.2016.90
Ghosh MK, K-hE C, Dill-Garlow R, Ma LJ, Yonezawa T, Itoh Y, Rivera L, Radecki KC, Wu QP, Arnold AP, Muller HK, Walker AM (2021) Sex differences in the immune system become evident in the perinatal period in the four core genotypes mouse. Front Endocrinol (Lausanne). https://doi.org/10.3389/fendo.2021.582614
Christoforidou Z, Mora Ortiz M, Poveda C, Abbas M, Walton G, Bailey M, Lewis MC (2019) Sexual dimorphism in immune development and in response to nutritional intervention in neonatal piglets. Front Immunol 10:2705. https://doi.org/10.3389/fimmu.2019.02705
Berghöfer B, Frommer T, Haley G, Fink L, Bein G, Hackstein H (2006) TLR7 ligands induce higher IFN-α production in females. J Immunol 177(4):2088–2096. https://doi.org/10.4049/jimmunol.177.4.2088
Griesbeck M, Ziegler S, Laffont S, Smith N, Chauveau L, Tomezsko P, Sharei A, Kourjian G, Porichis F, Hart M, Palmer CD, Sirignano M, Beisel C, Hildebrandt H, Cenac C, Villani AC, Diefenbach TJ, Le Gall S, Schwartz O, Herbeuval JP, Autran B, Guery JC, Chang JJ, Altfeld M (2015) Sex differences in plasmacytoid dendritic cell levels of IRF5 drive higher IFN- production in women. J Immunol 195(11):5327–5336. https://doi.org/10.4049/jimmunol.1501684
Schnyder-Candrian S, Togbe DE, Couillin I, Mercier I, Brombacher F, Quesniaux VR, Fossiez F, Ryffel B, Schnyder B (2006) Interleukin-17 is a negative regulator of established allergic asthma. J Exp Med 203(12):2715–2725. https://doi.org/10.1084/jem.20061401
Razzaghian HR, Sharafian Z, Sharma AA, Boyce GK, Lee K, Da Silva R, Orban PC, Sekaly R-P, Ross CJ, Lavoie PM (2021) Neonatal T helper 17 responses are skewed towards an immunoregulatory interleukin-22 phenotype. Front Immunol. https://doi.org/10.3389/fimmu.2021.655027
Dhuban KB, D’Hennezel E, Ben-Shoshan M, McCusker C, Clarke A, Fiset P, Mazer B, Piccirillo CA (2013) Altered T helper 17 responses in children with food allergy. Int Arch Allergy Immunol 162(4):318–322. https://doi.org/10.1159/000354028
Monk JM, Hou TY, Turk HF, McMurray DN, Chapkin RS (2013) n3 PUFAs reduce mouse CD4+ T cell ex vivo polarization into Th17 cells. J Nutr 143(9):1501–1508. https://doi.org/10.3945/jn.113.178178
Brown K, Decoffe D, Molcan E, Gibson DL (2012) Diet-induced dysbiosis of the intestinal microbiota and the effects on immunity and disease. Nutrients 4(8):1095–1119. https://doi.org/10.3390/nu4081095
Cebra JJ, Jiang H-Q, Boiko N, Tlaskalova-Hogenova H (2005) Chapter 18—the role of mucosal microbiota in the development, maintenance, and pathologies of the mucosal immune system. In: Mestecky J, Lamm ME, McGhee JR, Bienenstock J, Mayer L, Strober W (eds) Mucosal immunology, 3rd edn. Academic Press, Burlington, pp 335–368. https://doi.org/10.1016/B978-012491543-5/50022-X
Acknowledgements
We would like to thank Nuseed for providing the DHA-enriched canola and DSM for providing the ARAsco oils used in preparing the animal diets. The authors would like to express gratitude towards help received from everyone involved in the completion of the project. Stephanie Tollenaar helped with animal care and handling. The summer students João Vitor Wagner and Alan Liang assistant with tissue collection, processing and laboratory procedures. Senior laboratory members Dr. Jessy Azarcoya and Dr. Marnie Newell provided technical assistance and analysis throughout the project.
Funding
This study was funded by Natural Sciences and Engineering Research Council discovery grant (NSERC RGPIN-2017-04746) to CJF and a grant from the Alberta Canola Producers Commission. DP has received scholarships from AGES ALES University of Alberta. DP has also received awards from GSS Government of Alberta and the 2020 Fisher Scientific Graduate Scholarship for scholarly achievements.
Author information
Authors and Affiliations
Contributions
DP, SG, CR and CJF designed the study. DP, SG and JM conducted the research and analysed data. DP performed the statistical analysis and drafted the manuscript with the supervision of CJF, CR and ST. CJF has main responsibility for the final draft. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests in relation to the study.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Patel, D., Munhoz, J., Goruk, S. et al. Maternal diet supplementation with high-docosahexaenoic-acid canola oil, along with arachidonic acid, promotes immune system development in allergy-prone BALB/c mouse offspring at 3 weeks of age. Eur J Nutr 62, 2399–2413 (2023). https://doi.org/10.1007/s00394-023-03160-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-023-03160-6