Abstract
Purpose
Recent studies indicate that dysbiosis of gut microbiota and low-grade inflammation are important pathogenic determinants of type two diabetes mellitus (T2DM). The aim of this study is to investigate the effects of Lactobacillus GG on glycemic control, lipid profile, inflammatory parameters, and some gene expression levels in individuals with T2DM.
Methods
In a randomized, placebo-controlled trial, 34 women, aged 30–60 years with T2DM consumed daily probiotics or placebo for 8 weeks. The probiotic group consumed 10 × 109 Cfu/day Lactobacillus rhamnosus GG ATCC 53,103 (LGG), approved by the TR Ministry of Food, Agriculture, and Livestock. Anthropometric measurements, food diary, fasting blood, and fecal samples were taken at baseline and post-treatment.
Results
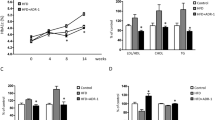
Fasting blood glucose was significantly decreased in probiotic (p = 0.049) and placebo (p = 0.028), but there was no difference between the groups. In the probiotic group, no significant difference was observed in HbA1c, fructosamine, lipid profile, and inflammatory variables compared to baseline. In this group, with LGG supplementation, mucin 2 and 3A (MUC2 and MUC3A) gene expressions increased more than ninefolds (p = 0.046 and p = 0.008, respectively) at post-treatment. Meanwhile, there was no significant change in any of the gene expressions in the placebo group. There was no significant difference in energy, protein, dietary fiber, and cholesterol intakes between placebo and probiotic groups during the study. However, daily fat intake (p = 0.003), body weight (p = 0.014), and body fat (p = 0.015) in the probiotic group were significantly decreased.
Conclusion
In this study, the effects of a single probiotic strain were investigated for 8 weeks. At the end of the study, although there was no finding that clearly reflected on the glycemic parameters of T2DM, its beneficial effects on the expression of mucin genes, which are responsible for weight loss and protection of intestinal barrier functions, cannot be denied. Further studies are needed to reveal the importance of these findings.
Clinical trial registration
ID: NCT05066152, October 4, 2021 retrospectively registered in ClinicalTrials.gov PRS web site.

Similar content being viewed by others
Data availability
The datasets generated during and analyzed during the current study are available from the corresponding author upon reasonable request.
References
IDF Diabetes Atlas 10th edition 2021 (2021) https://diabetesatlas.org Accessed 9 June 2022
Satman I, Omer B, Tutuncu Y, Kalaca S, Gedik S, Dinccag N et al (2013) Twelve-year trends in the prevalence and risk factors of diabetes and prediabetes in Turkish adults (TURDEP-II). Eur J Epidemiol 28(2):169–180. https://doi.org/10.1007/s10654-013-9771-5
Larsen N, Vogensen FK, Berg FWJ, Nielsen DS, Andreasen AS, Pedersen BK et al (2010) Gut Microbiota in Human Adults with Type 2 Diabetes Differs from Non-Diabetic Adults. PLoS ONE 5(2):e9085. https://doi.org/10.1371/journal.pone.0009085
Mansour SR, Moustafa MAA, Saad BM, Hamed R, Moustafa AA (2021) Impact of diet on human gut microbiome and disease risk. New Microbes New Infect 41:100845. https://doi.org/10.1016/j.nmni.2021.100845
Gomes AC, Bueno AA, Souza RG, Mota JF (2014) Gut microbiota, probiotics and diabetes. Nutr J 13(60):1–13. https://doi.org/10.1186/1475-2891-13-60
Lee JY, Hwang DH (2006) The modulation of inflammatory gene expression by lipids: mediation through toll-like receptors. Mol Cells 21:174–185
Purwanta MLA, Dewi NPAP, Saraswati MR (2017) Probiotics for type 2 diabetes mellitus: an anti-diabetic intervention to see beyond the gut. Indones J Biomed Sci 11:11–18. https://doi.org/10.15562/IJBS.V11I2.139
Li C, Li X, Han H, Cui H, Peng M, Wang G et al (2016) Effect of probiotics on metabolic profiles in type 2 diabetes mellitus: A meta-analysis of randomized, controlled trials. Medicine (Baltimore) 95(26):e4088. https://doi.org/10.1097/MD.0000000000004088
Paone P, Cani P (2020) Mucus barrier, mucins and gut microbiota: the expected slimy partners? Gut 69(12):2232–2243. https://doi.org/10.1136/gutjnl-2020-322260
Liu Y, Yu X, Zhao J, Zhang H, Zhai Q, Chen W (2020) The role of MUC2 mucin in intestinal homeostasis and the impact of dietary components on MUC2 expression. Int J Biol Macromol 164:884–891. https://doi.org/10.1016/j.ijbiomac.2020.07.191
Gan GL, Liu J, Chen WJ, Ye QQ, Xu Y, Wu HT, Li W (2020) The diverse roles of the mucin gene cluster located on chromosome 11p15.5 in colorectal cancer. Front Cell Dev Biol 8:514. https://doi.org/10.3389/fcell.2020.00514
Su W, Feng B, Hu L, Guo X, Yu M (2022) MUC3A promotes the progression of colorectal cancer through the PI3K/Akt/mTOR pathway. BMC Cancer 22(1):602. https://doi.org/10.1186/s12885-022-09709-8
Mack DR (2005) Probiotics-mixed messages. Can Fam Physician 51(11):1455–1464
Doron S, Snydman DR, Gorbach SL (2005) Lactobacillus GG: bacteriology and clinical applications. Gastroenterol Clin North Am 34:483–498. https://doi.org/10.1016/j.gtc.2005.05.011
Capurso L (2019) Thirty Years of Lactobacillus rhamnosus GG: a Review. J Clin Gastroenterol 53(Suppl 1):S1–S41. https://doi.org/10.1097/MCG.0000000000001170
Tabuchi M, Ozaki M, Tamura A, Yamada N, Ishida T, Hosoda M et al (2003) Antidiabetic effect of Lactobacillus GG in streptozotocin-induced diabetic rats. Biosci Biotechnol Biochem 67(6):1421–1424. https://doi.org/10.1271/bbb.67.1421
Honda K, Moto M, Uchida N, He F, Hashizume N (2012) Anti-diabetic effects of lactic acid bacteria in normal and type 2 diabetic mice. J Clin Biochem Nutr 51(2):96–101. https://doi.org/10.3164/jcbn.11-07
Liu S, Wang X, Kai Y, Tian C, Guo S, He L, Zhai D, Song X (2022) Clinical significance of high mobility group box 1/toll-like receptor 4 in obese diabetic patients. Endocr J 69(3):235–242. https://doi.org/10.1507/endocrj.EJ21-0381
Jang S, Lakshman S, Beshah E et al (2017) Flavanol-rich cocoa powder interacts with lactobacillus rhamnossus LGG to alter the antibody response to infection with the parasitic nematode ascaris suum. Nutrients 9(10):1113. https://doi.org/10.3390/nu9101113
Khailova L, Baird CH, Rush AA, Barnes C, Wischmeyer PE (2017) Lactobacillus rhamnosus GG treatment improves intestinal permeability and modulates inflammatory response and homeostasis of spleen and colon in experimental model of Pseudomonas aeruginosa pneumonia. Clin Nutr 36(6):1549–1557. https://doi.org/10.1016/j.clnu.2016.09.025
Andreasen AS, Larsen N, Pedersen-Skovsgaard T, Berg RM, Møller K, Svendsen KD et al (2010) Effects of Lactobacillus acidophilus NCFM on insulin sensitivity and the systemic inflammatory response in human subjects. Br J Nutr 104(12):1831–1838. https://doi.org/10.1017/S0007114510002874
Mazloom Z, Yousefinejad A, Dabbaghmanesh MH (2013) Effect of probiotics on lipid profile, glycemic control, insulin action, oxidative stress, and inflammatory markers in patients with type 2 diabetes: a clinical trial. Iran J Med Sci 38(1):38–43
Moroti C, Souza Magri LF, de Rezende CM, Cavallini DC, Sivieri K (2012) Effect of the consumption of a new symbiotic shake on glycemia and cholesterol levels in elderly people with type 2 diabetes mellitus. Lipids Health Dis 11:29. https://doi.org/10.1186/1476-511X-11-29
Tonucci LB, Olbrich Dos Santos KM, Licursi de Oliveira L, Rocha Ribeiro SM, Duarte Martino HS (2017) Clinical application of probiotics in type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled study. Clin Nutr 36(1):85–92. https://doi.org/10.1016/j.clnu.2015.11.011
Asemi Z, Zare Z, Shakeri H, Sabihi SS, Esmaillzadeh A (2013) Effect of multispecies probiotic supplements on metabolic profiles, hs-CRP, and oxidative stress in patients with type 2 diabetes. Ann Nutr Metab 63(1–2):1–9. https://doi.org/10.1159/000349922
Sanborn VE, Azcarate-Peril MA, Gunstad J (2020) Lactobacillus rhamnosus GG and HbA1c in middle age and older adults without type 2 diabetes mellitus: a preliminary randomized study. Diabetes Metab Syndr 14(5):907–909. https://doi.org/10.1016/j.dsx.2020.05.034
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28(7):412–419. https://doi.org/10.1007/BF00280883
Friedewald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18(6):499–502
Chen H, Sullivan G, Yue LQ, Katz A, Quon MJ (2003) QUICKI is a useful index of insulin sensitivity in subjects with hypertension. Am J Physiol Endocrinol Metab 284(4):E804–E812. https://doi.org/10.1152/ajpendo.00330.2002
World Health Organization (2008) Waist circumference and waist-hip ratio: Report of a WHO expert consultation ISBN 978 92 4 150149 1. http://www.who.int/about/licensing/copyright_form/en/index.html. Accessed on Aug 29 2021
Bebispro for Windows, Stuttgart, Germany; Turkish Version (BeBiS 8.2), (2019) Pasifik Elektirik Elektronik Ltd. Şti. (www.bebis.com.tr); Istanbul
Otte JM, Podolsky DK (2004) Functional modulation of enterocytes by gram-positive and gram-negative microorganisms. Am J Physiol Gastrointest Liver Physiol 286(4):G613–G626. https://doi.org/10.1152/ajpgi.00341.2003
Mack DR, Michail S, Wei S, McDougall L, Hollingsworth MA (1999) Probiotics inhibit enteropathogenic E. coli adherence in vitro by inducing intestinal mucin gene expression. Am J Physiol 276(4):G941–G950. https://doi.org/10.1152/ajpgi.1999.276.4.G941
Gaudier E, Michel C, Segain JP, Cherbut C, Hoebler C (2005) The VSL# 3 probiotic mixture modifies microflora but does not heal chronic dextran-sodium sulfate-induced colitis or reinforce the mucus barrier in mice. J Nutr 135(12):2753–2761. https://doi.org/10.1093/jn/135.12.2753
Caballero-Franco C, Keller K, De Simone C, Chadee K (2007) The VSL#3 probiotic formula induces mucin gene expression and secretion in colonic epithelial cells. Am J Physiol Gastrointest Liver Physiol 292(1):G315–G322. https://doi.org/10.1152/ajpgi.00265.2006
Ejtahed HS, Mohtadi-Nia J, Homayouni-Rad A, Niafar M, Asghari-Jafarabadi M, Mofid V et al (2011) Effect of probiotic yogurt containing Lactobacillus acidophilus and Bifidobacterium lactis on lipid profile in individuals with type 2 diabetes mellitus. J Dairy Sci 94(7):3288–3294. https://doi.org/10.3168/jds.2010-4128
Ejtahed HS, Mohtadi-Nia J, Homayouni-Rad A, Niafar M, Asghari-Jafarabadi M, Mofid V (2012) Probiotic yogurt improves antioxidant status in type 2 diabetic patients. Nutrition 28(5):539–543. https://doi.org/10.1016/j.nut.2011.08.013
Firouzi S, Majid HA, Ismail A, Kamaruddin NA, Barakatun-Nisak MY (2017) Effect of multi-strain probiotics (multi-strain microbial cell preparation) on glycemic control and other diabetes-related outcomes in people with type 2 diabetes: a randomized controlled trial. Eur J Nutr 56(4):1535–1550. https://doi.org/10.1007/s00394-016-1199-8
Asemi Z, Bahmani S, Shakeri H, Jamal A, Faraji AM (2015) Effect of multispecies probiotic supplements on serum minerals, liver enzymes and blood pressure in patients with type 2 diabetes. Int J Diabetes Dev Ctries 35(2):90–95. https://doi.org/10.1007/s13410-013-0187-2
Sanchez M, Darimont C, Drapeau V et al (2014) Effect of Lactobacillus rhamnosus CGMCC1.3724 supplementation on weight loss and maintenance in obese men and women. Br J Nutr 111(8):1507–1519. https://doi.org/10.1017/S0007114513003875
Kim SW, Park KY, Kim B, Kim E, Hyun CK (2013) Lactobacillus rhamnosus GG improves insulin sensitivity and reduces adiposity in high-fat diet-fed mice through enhancement of adiponectin production. Biochem Biophys Res Commun 431(2):258–263. https://doi.org/10.1016/j.bbrc.2012.12.121
Asemi Z, Khorrami-Rad A, Alizadeh SA, Shakeri H, Esmaillzadeh A (2014) Effects of synbiotic food consumption on metabolic status of diabetic patients: a double-blind randomized cross-over controlled clinical trial. Clin Nutr 33(2):198–203. https://doi.org/10.1016/j.clnu.2013.05.015
Ostadrahimi A, Taghizadeh A, Mobasseri M, Farrin N, Payahoo L, Gheshlaghi BZ et al (2015) Effect of probiotic fermented milk (kefir) on glycemic control and lipid profile in type 2 diabetic patients: a randomized double-blind placebo-controlled clinical trial. Iran J Public Health 44(2):228–237
Mohamadshahi M, Veissi M, Haidari F, Javid AZ, Mohammadi F, Shirbeigi E (2014) Effects of probiotic yogurt consumption on lipid profile in type 2 diabetic patients: a randomized controlled clinical trial. J Res Med Sci 19(6):531–536
Mohamadshahi M, Veissi M, Haidari F, Shahbazian H, Kaydani GA, Mohammadi F (2014) Effects of probiotic yogurt consumption on inflammatory biomarkers in patients with type 2 diabetes. Bioimpacts 4(2):83–88. https://doi.org/10.5681/bi.2014.007
Farrokhian A, Raygan F, Soltani A, Tajabadi-Ebrahimi M, Sharifi Esfahani M, Karami AA et al (2019) The effects of synbiotic supplementation on carotid intima-media thickness, biomarkers of inflammation, and oxidative stress in people with overweight, diabetes, and coronary heart disease: a randomized, double-blind. Plasebo-Controlled Trial Probiotics Antimicrob Proteins 11(1):133–142. https://doi.org/10.1007/s12602-017-9343-1
Ahmad R, Al-Mass A, Atizado V, Al-Hubail A, Al-Ghimlas F, Al-Arauj M et al (2012) Elevated expression of the toll like receptors 2 and 4 in obese individuals: its significance for obesity-induced inflammation. J Inflamm (Lond) 9(1):48. https://doi.org/10.1186/1476-9255-9-48
Dasu MR, Devaraj S, Park S, Jialal I (2010) Increased toll-like receptor (TLR) activation and TLR ligands in recently diagnosed type 2 diabetic subjects. Diabetes Care 33(4):861–868. https://doi.org/10.2337/dc09-1799
Acknowledgements
The authors are grateful to the Menarini Co, Türkiye for providing probiotic products. The authors are also thankful to Zade Vital Co. for preparing placebo corn oil.
Funding
The present work was supported by the Scientific Research Fund of Istanbul University. Project no. 57789.
Author information
Authors and Affiliations
Contributions
BET: conceptualization, funding acquisition, methodology, investigation, writing—original draft. EC: funding acquisition, methodology, formal analysis, investigation, resources, writing—review and editing. DA and BO: formal analysis. PC: project administration, formal analysis. SP: formal analysis supervision, resources. IS: project administration, resources, interpreting the results, writing—review and editing. FA: supervision, project administration, writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Eliuz Tipici, B., Coskunpinar, E., Altunkanat, D. et al. Lactobacillus GG is associated with mucin genes expressions in type 2 diabetes mellitus: a randomized, placebo-controlled trial. Eur J Nutr 62, 2155–2164 (2023). https://doi.org/10.1007/s00394-023-03139-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-023-03139-3