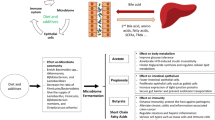
Abstract
Purpose
High-fat diets have different metabolic responses via gut dysbiosis. In this review, we discuss the complex interaction between the intake of long- and medium-chain saturated fatty acids (SFAs), gut microbiota, and white adipose tissue (WAT) dysfunction, particularly focusing on the type of fat.
Results
The evidence for the impact of dietary SFAs on the gut microbiota–WAT axis has been mostly derived from in vitro and animal models, but there is now also evidence emerging from human studies. Most current reports show that, in response to high long- and medium-chain SFA diets, WAT functions are altered and can be modulated from microbial metabolites in several manners; and it appears to be also modified under conditions of obesity. SFAs overconsumption can reduce bacterial content and disrupt the gut environment. Both long- and medium-chain SFAs may contribute to proinflammatory cytokines release and TLR4 cascade signaling, either by regulation of endotoxemia markers or myristoylated protein. Palmitic and stearic acids have pathological effects on the intestinal epithelium, microbes, and inflammatory and lipogenic WAT profiles. While myristic and lauric acids display somewhat controversial outcomes, from probiotic effects and contribution to weight loss to cardiometabolic alterations from WAT inflammation.
Conclusion
Identifying an interference of distinct types of SFA in the binomial gut microbiota–WAT may elucidate essential mechanisms of metabolic endotoxemia, which may be the key to triggering obesity, innovating the therapeutic tools for this disease.


Similar content being viewed by others
Availability of data and materials
None to disclosure.
References
de Carvalho CCCR, Caramujo MJ (2018) The various roles of fatty acids. Molecules 23:2583. https://doi.org/10.3390/molecules23102583
Calder PC (2011) Fatty acids and inflammation: the cutting edge between food and pharma. Eur J Pharmacol 668:S50–S58. https://doi.org/10.1016/j.ejphar.2011.05.085
Perfilyev A, Dahlman I, Gillberg L et al (2017) Impact of polyunsaturated and saturated fat overfeeding on the DNA-methylation pattern in human adipose tissue: a randomized controlled trial. Am J Clin Nutr 105:991–1000. https://doi.org/10.3945/ajcn.116.143164. Erratum in: Am J Clin Nutr (2017) 106:325
Soni N, Ross AB, Scheers N et al (2019) The omega-3 fatty acids EPA and DHA, as a part of a murine high-fat diet, reduced lipid accumulation in brown and white adipose tissues. Int J Mol Sci 20:5895. https://doi.org/10.3390/ijms20235895
Anhê FF, Jensen BAH, Varin TV et al (2020) Type 2 diabetes influences bacterial tissue compartmentalisation in human obesity. Nat Metab 2:233–242. https://doi.org/10.1038/s42255-020-0178-9
Massier L, Chakaroun R, Tabei S et al (2020) Adipose tissue derived bacteria are associated with inflammation in obesity and type 2 diabetes. Gut 69:1796–1806. https://doi.org/10.1136/gutjnl-2019-320118
Lancaster GI, Langley KG, Berglund NA et al (2018) Evidence that TLR4 is not a receptor for saturated fatty acids but mediates lipid-induced inflammation by reprogramming macrophage metabolism. Cell Metab 27:1096-1110.e5. https://doi.org/10.1016/j.cmet.2018.03.014
Vaittinen M, Männistö V, Käkelä P et al (2017) Interorgan cross talk between fatty acid metabolism, tissue inflammation, and FADS2 genotype in humans with obesity. Obesity (Silver Spring) 25:545–552. https://doi.org/10.1002/oby.21753
Moreno-Navarrete JM, Fernandez-Real JM (2019) The gut microbiota modulates both browning of white adipose tissue and the activity of brown adipose tissue. Rev Endocr Metab Disord 20:387–397. https://doi.org/10.1007/s11154-019-09523-x
Ralston JC, Lyons CL, Kennedy EB et al (2017) Fatty acids and NLRP3 inflammasome-mediated inflammation in metabolic tissues. Annu Rev Nutr 37:77–102. https://doi.org/10.1146/annurev-nutr-071816-064836
Velloso LA, Folli F, Saad MJ (2015) TLR4 at the crossroads of nutrients, gut microbiota, and metabolic inflammation. Endocr Rev 36:245–271. https://doi.org/10.1210/er.2014-1100
Cani PD, Knauf C (2016) How gut microbes talk to organs: the role of endocrine and nervous routes. Mol Metab 5:743–752. https://doi.org/10.1016/j.molmet.2016.05.011
Wells JM, Brummer RJ, Derrien M et al (2017) Homeostasis of the gut barrier and potential biomarkers. Am J Physiol Gastrointest Liver Physiol 312:G171–G193. https://doi.org/10.1152/ajpgi.00048.2015
Jamar G, Ribeiro DA, Pisani LP (2020) High-fat or high-sugar diets as trigger inflammation in the microbiota-gut-brain axis. Crit Rev Food Sci Nutr 8:1–19. https://doi.org/10.1080/10408398.2020.1747046
Araújo JR, Tomas J, Brenner C et al (2017) Impact of high-fat diet on the intestinal microbiota and small intestinal physiology before and after the onset of obesity. Biochimie 141:97–106. https://doi.org/10.1016/j.biochi.2017.05.019
Cani PD (2019) Microbiota and metabolites in metabolic diseases. Nat Rev Endocrinol 15:69–70. https://doi.org/10.1038/s41574-018-0143-9
Hersoug LG, Møller P, Loft S (2018) Role of microbiota-derived lipopolysaccharide in adipose tissue inflammation, adipocyte size and pyroptosis during obesity. Nutr Res Rev 31:153–163. https://doi.org/10.1017/S0954422417000269
Meilhac O, Tanaka S, Couret D (2020) High-density lipoproteins are bug scavengers. Biomolecules 10:598. https://doi.org/10.3390/biom10040598
Laugerette F, Alligier M, Bastard JP et al (2014) Overfeeding increases postprandial endotoxemia in men: Inflammatory outcome may depend on LPS transporters LBP and sCD14. Mol Nutr Food Res 58:1513–1518. https://doi.org/10.1002/mnfr.201400044
Caesar R, Tremaroli V, Kovatcheva-Datchary P et al (2015) Crosstalk between Gut Microbiota and Dietary Lipids Aggravates WAT Inflammation through TLR Signaling. Cell Metab 22:658–668. https://doi.org/10.1016/j.cmet.2015.07.026
Genser L, Aguanno D, Soula HA et al (2018) Increased jejunal permeability in human obesity is revealed by a lipid challenge and is linked to inflammation and type 2 diabetes. J Pathol 246:217–230. https://doi.org/10.1002/path.5134
Schugar RC, Willard B, Wang Z et al (2018) Postprandial gut microbiota-driven choline metabolism links dietary cues to adipose tissue dysfunction. Adipocyte 7:49–56. https://doi.org/10.1080/21623945.2017.1398295
Zeng H, Umar S, Rust B et al (2019) Secondary bile acids and short chain fatty acids in the colon: a focus on colonic microbiome, cell proliferation, inflammation, and cancer. Int J Mol Sci 20:1214. https://doi.org/10.3390/ijms20051214
Holmes AJ, Chew YV, Colakoglu F et al (2017) Diet–microbiome interactions in health are controlled by intestinal nitrogen source constraints. Cell Metab 25:140–151. https://doi.org/10.1016/j.cmet.2016.10.021
Yoo W, Zieba JK, Foegeding NJ et al (2021) High-fat diet-induced colonocyte dysfunction escalates microbiota-derived trimethylamine N-oxide. Science 373:813–818. https://doi.org/10.1126/science.aba3683
Moran AW, Daly K, Al-Rammahi MA et al (2020) Nutrient sensing of gut luminal environment. Proc Nutr Soc. https://doi.org/10.1017/S0029665120007120
Dirksen C, Graff J, Fuglsang S et al (2019) Energy intake, gastrointestinal transit, and gut hormone release in response to oral triglycerides and fatty acids in men with and without severe obesity. Am J Physiol Gastrointest Liver Physiol 316:G332–G337. https://doi.org/10.1152/ajpgi.00310.2018
Husted AS, Trauelsen M, Rudenko O et al (2017) GPCR-mediated signaling of metabolites. Cell Metab 25:777–796. https://doi.org/10.1016/j.cmet.2017.03.008
Wanders D, Graff EC, Judd RL (2012) Effects of high fat diet on GPR109A and GPR81 gene expression. Biochem Biophys Res Commun 425:278–283. https://doi.org/10.1016/j.bbrc.2012.07.082
Amisten S, Neville M, Hawkes R et al (2015) An atlas of G-protein coupled receptor expression and function in human subcutaneous adipose tissue. Pharmacol Ther 146:61–93. https://doi.org/10.1016/j.pharmthera.2014.09.007
Dewulf EM, Cani PD, Neyrinck AM et al (2011) Inulin-type fructans with prebiotic properties counteract GPR43 overexpression and PPARγ-related adipogenesis in the white adipose tissue of high-fat diet-fed mice. J Nutr Biochem 22:712–722. https://doi.org/10.1016/j.jnutbio.2010.05.009
Nagasaki H, Kondo T, Fuchigami M et al (2012) Inflammatory changes in adipose tissue enhance expression of GPR84, a medium-chain fatty acid receptor: TNFα enhances GPR84 expression in adipocytes. FEBS Lett 586:368–372. https://doi.org/10.1016/j.febslet.2012.01.001 (Epub 2012 Jan 10)
Nakajima A, Nakatani A, Hasegawa S et al (2017) The short chain fatty acid receptor GPR43 regulates inflammatory signals in adipose tissue M2-type macrophages. PLoS ONE 12:e0179696. https://doi.org/10.1371/journal.pone.0179696
Vieira AT, Macia L, Galvão I et al (2015) A role for gut microbiota and the metabolite-sensing receptor GPR43 in a murine model of gout. Arthritis Rheumatol 67:1646–1656. https://doi.org/10.1002/art.39107
Sivaprakasam S, Prasad PD, Singh N (2016) Benefits of short-chain fatty acids and their receptors in inflammation and carcinogenesis. Pharmacol Ther 164:144–151. https://doi.org/10.1016/j.pharmthera.2016.04.007
Hajishengallis G, Lambris JD (2016) More than complementing Tolls: complement-Toll-like receptor synergy and crosstalk in innate immunity and inflammation. Immunol Rev 274:233–244. https://doi.org/10.1111/imr.12467
Mobraten K, Haugbro T, Karlstrom E et al (2015) Activation of the bile acid receptor TGR5 enhances LPS-induced inflammatory responses in a human monocytic cell line. J Recept Signal Transduct Res 35:402–409. https://doi.org/10.3109/10799893.2014.986744
Canfora EE, Jocken JW, Blaak EE (2015) Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol 11:577–591. https://doi.org/10.1038/nrendo.2015.128
Tazi A, Araujo JR, Mulet C et al (2018) Disentangling host-microbiota regulation of lipid secretion by enterocytes: insights from commensals Lactobacillus paracasei and Escherichia coli. MBio 9:e01493-e1518. https://doi.org/10.1128/mBio.01493-18
Gunness P, Williams BA, Gerrits WJ et al (2016) Circulating triglycerides and bile acids are reduced by a soluble wheat arabinoxylan via modulation of bile concentration and lipid digestion rates in a pig model. Mol Nutr Food Res 60:642–651. https://doi.org/10.1002/mnfr.201500686
Schoeler M, Caesar R (2019) Dietary lipids, gut microbiota and lipid metabolism. Rev Endocr Metab Disord 20:461–472. https://doi.org/10.1007/s11154-019-09512-0
Wan Y, Yuan J, Li J et al (2020) Unconjugated and secondary bile acid profiles in response to higher-fat, lower-carbohydrate diet and associated with related gut microbiota: a 6-month randomized controlled-feeding trial. Clin Nutr 39:395–404. https://doi.org/10.1016/j.clnu.2019.02.037
Fiorucci S, Distrutti E (2015) Bile acid-activated receptors, intestinal microbiota, and the treatment of metabolic disorders. Trends Mol Med 21:702–714. https://doi.org/10.1016/j.molmed.2015.09.001
Chávez-Talavera O, Tailleux A, Lefebvre P et al (2017) bile acid control of metabolism and inflammation in obesity, type 2 diabetes, dyslipidemia, and nonalcoholic fatty liver disease. Gastroenterology 152:1679-1694.e3. https://doi.org/10.1053/j.gastro.2017.01.055
Wei M, Huang F, Zhao L et al (2020) A dysregulated bile acid-gut microbiota axis contributes to obesity susceptibility. EBioMedicine 55:102766. https://doi.org/10.1016/j.ebiom.2020.102766
Parséus A, Sommer N, Sommer F et al (2017) Microbiota-induced obesity requires farnesoid X receptor. Gut 66:429–437. https://doi.org/10.1136/gutjnl-2015-310283
Schmid A, Schlegel J, Thomalla M et al (2019) Evidence of functional bile acid signaling pathways in adipocytes. Mol Cell Endocrinol 483:1–10. https://doi.org/10.1016/j.mce.2018.12.006
Jia W, Xie G, Jia W et al (2018) Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat Rev Gastroenterol Hepatol 15:111–128. https://doi.org/10.1038/nrgastro.2017.119
Svensson PA, Olsson M, Andersson-Assarsson JC et al (2013) The TGR5 gene is expressed in human subcutaneous adipose tissue and is associated with obesity, weight loss and resting metabolic rate. Biochem Biophys Res Commun 433:563–566. https://doi.org/10.1016/j.bbrc.2013.03.031
Santos RD, Gagliardi AC, Xavier HT et al (2013) Diretriz sobre o consumo de gorduras e saúde cardiovascular [First guidelines on fat consumption and cardiovascular health]. Arq Bras Cardiol 100:1–40
Li B, Leung JCK, Chan LYY et al (2020) A global perspective on the crosstalk between saturated fatty acids and Toll-like receptor 4 in the etiology of inflammation and insulin resistance. Prog Lipid Res 77:101020. https://doi.org/10.1016/j.plipres.2019.101020
DiNicolantonio JJ, O’Keefe JH (2017) Good fats versus bad fats: a comparison of fatty acids in the promotion of insulin resistance, inflammation, and obesity. Mo Med 114:303–307
Coelho OGL, Cândido FG, Alfenas RCG (2019) Dietary fat and gut microbiota: mechanisms involved in obesity control. Crit Rev Food Sci Nutr 59:3045–3053. https://doi.org/10.1080/10408398.2018.1481821
Rohr MW, Narasimhulu CA, Rudeski-Rohr TA, Parthasarathy S (2020) Negative effects of a high-fat diet on intestinal permeability: a review. Adv Nutr 11:77–91. https://doi.org/10.1093/advances/nmz061
Wolters M, Ahrens J, Romaní-Pérez M et al (2019) Dietary fat, the gut microbiota, and metabolic health—a systematic review conducted within the MyNewGut project. Clin Nutr 38:2504–2520. https://doi.org/10.1016/j.clnu.2018.12.024
Mokkala K, Houttu N, Cansev T, Laitinen K (2020) Interactions of dietary fat with the gut microbiota: evaluation of mechanisms and metabolic consequences. Clin Nutr 39:994–1018. https://doi.org/10.1016/j.clnu.2019.05.003
Basak S, Banerjee A, Pathak S, Duttaroy AK (2022) Dietary fats and the gut microbiota: their impacts on lipid-induced metabolic syndrome. J Funct Foods 91:105026. https://doi.org/10.1016/j.jff.2022.105026
Sze MA, Schloss PD (2016) Looking for a signal in the noise: revisiting obesity and the microbiome. mBio 7:e01018-16. https://doi.org/10.1128/mBio.01018-16. Erratum in: MBio (2017) 8(6)
Xu AA, Kennedy LK, Hoffman K et al (2022) Dietary fatty acid intake and the colonic gut microbiota in humans. Nutrients 14:2722. https://doi.org/10.3390/nu14132722
Fava F, Gitau R, Griffin BA et al (2013) The type and quantity of dietary fat and carbohydrate alter faecal microbiome and short-chain fatty acid excretion in a metabolic syndrome “at-risk” population. Int J Obes (Lond) 37:216–223. https://doi.org/10.1038/ijo.2012.33
Wan Y, Wang F, Yuan J et al (2019) Effects of dietary fat on gut microbiota and faecal metabolites, and their relationship with cardiometabolic risk factors: a 6-month randomised controlled-feeding trial. Gut 68:1417–1429. https://doi.org/10.1136/gutjnl-2018-317609
Bailén M, Bressa C, Martínez-López S et al (2020) Microbiota features associated with a high-fat/low-fiber diet in healthy adults. Front Nutr 7:583608. https://doi.org/10.3389/fnut.2020.583608
Mazaki-Tovi M, Bolin SR, Schenck PA (2018) Dietary fatty acids differentially regulate secretion of adiponectin and interleukin-6 in primary canine adipose tissue culture. Lipids 53:205–216. https://doi.org/10.1002/lipd.12021
Mazaki-Tovi M, Bolin SR, Schenck PA (2019) Adipokines secretion in feline primary adipose tissue culture in response to dietary fatty acids. BMC Vet Res 15:324. https://doi.org/10.1186/s12917-019-2065-8
de Wit N, Derrien M, Bosch-Vermeulen H et al (2012) Saturated fat stimulates obesity and hepatic steatosis and affects gut microbiota composition by an enhanced overflow of dietary fat to the distal intestine. Am J Physiol Gastrointest Liver Physiol 303:G589–G599. https://doi.org/10.1152/ajpgi.00488.2011
Ye Z, Xu YJ, Liu Y (2021) Different typical dietary lipid consumption affects the bile acid metabolism and the gut microbiota structure: an animal trial using Sprague-Dawley rats. J Sci Food Agric. https://doi.org/10.1002/jsfa.11661
Ghezzal S, Postal BG, Quevrain E et al (2020) Palmitic acid damages gut epithelium integrity and initiates inflammatory cytokine production. Biochim Biophys Acta Mol Cell Biol Lipids 1865:158530. https://doi.org/10.1016/j.bbalip.2019.158530
Kim SJ, Kim SE, Kim AR et al (2019) Dietary fat intake and age modulate the composition of the gut microbiota and colonic inflammation in C57BL/6J mice. BMC Microbiol 19:193. https://doi.org/10.1186/s12866-019-1557-9
Hotamisligil GS (2017) Inflammation, metaflammation and immunometabolic disorders. Nature 542:177–185. https://doi.org/10.1038/nature21363
McNelis JC, Olefsky JM (2014) Macrophages, immunity, and metabolic disease. Immunity 41:36–48. https://doi.org/10.1016/j.immuni.2014.05.010
Wei X, Song H, Yin L et al (2016) Fatty acid synthesis configures the plasma membrane for inflammation in diabetes. Nature 539:294–298. https://doi.org/10.1038/nature20117
Laugerette F, Furet JP, Debard C et al (2012) Oil composition of high-fat diet affects metabolic inflammation differently in connection with endotoxin receptors in mice. Am J Physiol Endocrinol Metab 302:E374–E386. https://doi.org/10.1152/ajpendo.00314.2011
Laugerette F, Vors C, Géloën A et al (2011) Emulsified lipids increase endotoxemia: possible role in early postprandial low-grade inflammation. J Nutr Biochem 22:53–59. https://doi.org/10.1016/j.jnutbio.2009.11.011
Yang B, Zhang X, Gong H et al (2021) High stearic acid diet modulates gut microbiota and aggravates acute graft-versus-host disease. Signal Transduct Target Ther 6:277. https://doi.org/10.1038/s41392-021-00600-9
Zhao L, Huang Y, Lu L et al (2018) Saturated long-chain fatty acid-producing bacteria contribute to enhanced colonic motility in rats. Microbiome 6:107. https://doi.org/10.1186/s40168-018-0492-6
Tan R, Dong H, Chen Z et al (2021) Intestinal microbiota mediates high-fructose and high-fat diets to induce chronic intestinal inflammation. Front Cell Infect Microbiol 11:654074. https://doi.org/10.3389/fcimb.2021.654074
Kochumon S, Arefanian H, Azim R et al (2020) Stearic acid and TNF-α co-operatively potentiate MIP-1α production in monocytic cells via MyD88 independent TLR4/TBK/IRF3 signaling pathway. Biomedicines 8:403. https://doi.org/10.3390/biomedicines8100403
Małodobra-Mazur M, Cierzniak A, Pawełka D et al (2020) Metabolic differences between subcutaneous and visceral adipocytes differentiated with an excess of saturated and monounsaturated fatty acids. Genes (Basel) 11:1092. https://doi.org/10.3390/genes11091092
Boudry G, Hamilton MK, Chichlowski M et al (2017) Bovine milk oligosaccharides decrease gut permeability and improve inflammation and microbial dysbiosis in diet-induced obese mice. J Dairy Sci 100:2471–2481. https://doi.org/10.3168/jds.2016-11890
Huang EY, Leone VA, Devkota S et al (2013) Composition of dietary fat source shapes gut microbiota architecture and alters host inflammatory mediators in mouse adipose tissue. JPEN J Parenter Enteral Nutr 37:746–754. https://doi.org/10.1177/0148607113486931
O’Neill L, Pandya V, Grigoryan Z et al (2022) Effects of Milkfat on the gut microbiome of patients after bariatric surgery, a pilot study. Obes Surg 32:480–488. https://doi.org/10.1007/s11695-021-05805-z
Dao MC, Everard A, Clément K et al (2016) Losing weight for a better health: role for the gut microbiota. Clin Nutr Exp 6:39–58. https://doi.org/10.1016/j.yclnex.2015.12.001
Liu X, Mao B, Gu J et al (2021) Blautia-a new functional genus with potential probiotic properties? Gut Microbes 13:1–21. https://doi.org/10.1080/19490976.2021.1875796
Prieto I, Hidalgo M, Segarra AB et al (2018) Influence of a diet enriched with virgin olive oil or butter on mouse gut microbiota and its correlation to physiological and biochemical parameters related to metabolic syndrome. PLoS ONE 13:e0190368. https://doi.org/10.1371/journal.pone.0190368
Wang B, Dai T, Sun W et al (2021) Protein N-myristoylation: functions and mechanisms in control of innate immunity. Cell Mol Immunol 18:878–888. https://doi.org/10.1038/s41423-021-00663-2
Rowe DC, McGettrick AF, Latz E et al (2006) The myristoylation of TRIF-related adaptor molecule is essential for Toll-like receptor 4 signal transduction. Proc Natl Acad Sci USA 103:6299–6304. https://doi.org/10.1073/pnas.0510041103
Honda KL, Lamon-Fava S, Matthan NR et al (2015) EPA and DHA exposure alters the inflammatory response but not the surface expression of Toll-like receptor 4 in macrophages. Lipids 50:121–129. https://doi.org/10.1007/s11745-014-3971-y
Hubbard NE, Socolich RJ, Erickson KL (1996) Dietary myristic acid alters acylated proteins in activated murine macrophages. J Nutr 126:1563–1570. https://doi.org/10.1093/jn/126.6.1563
Laugerette F, Vors C, Alligier M et al (2020) Postprandial endotoxin transporters LBP and sCD14 differ in obese vs. overweight and normal weight men during fat-rich meal digestion. Nutrients 12:1820. https://doi.org/10.3390/nu12061820
Eyres L, Eyres MF, Chisholm A, Brown RC (2016) Coconut oil consumption and cardiovascular risk factors in humans. Nutr Rev 74:267–280. https://doi.org/10.1093/nutrit/nuw002
Zhou S, Wan Y, Jacoby J et al (2017) Effects of medium- and long-chain triacylglicerols on lipid metabolism and gut microbiota composition in C57BL/6L mice. J Agric Food Chem 65:6599–6607
Dias MM, Siqueira NP, Conceição LL et al (2018) Consumption of virgin coconut oil in Wistar rats increases saturated fatty acids in the liver and adipose tissue, as well as adipose tissue inflammation. J Funct Foods 48:472–480
Patrone V, Minuti A, Lizier M et al (2018) Differential effects of coconut versus soy oil on gut microbiota composition and predicted metabolic function in adult mice. BMC Genomics 19:808
López-Salazar V, Tapia MS, Tobón-Cornejo S et al (2021) Consumption of soybean or olive oil at recommended concentrations increased the intestinal microbiota diversity and insulin sensitivity and prevented fatty liver compared to the effects of coconut oil. J Nutr Biochem 94:108751. https://doi.org/10.1016/j.jnutbio.2021.108751
St-Onge MP, Bourque C, Jones PJ et al (2003) Medium- versus long-chain triglycerides for 27 days increases fat oxidation and energy expenditure without resulting in changes in body composition in overweight women. Int J Obes Relat Metab Disord 27:95–102. https://doi.org/10.1038/sj.ijo.0802169
St-Onge MP, Mayrsohn B, O’Keeffe M et al (2014) Impact of medium and long chain triglycerides consumption on appetite and food intake in overweight men. Eur J Clin Nutr 68:1134–1140. https://doi.org/10.1038/ejcn.2014.145
St-Onge MP, Ross R, Parsons WD et al (2003) Medium-chain triglycerides increase energy expenditure and decrease adiposity in overweight men. Obes Res 11:395–402. https://doi.org/10.1038/oby.2003.53
St-Onge MP, Bosarge A (2008) Weight-loss diet that includes consumption of medium-chain triacylglycerol oil leads to a greater rate of weight and fat mass loss than does olive oil. Am J Clin Nutr 87:621–626. https://doi.org/10.1093/ajcn/87.3.621
Vijayakumar M, Vasudevan DM, Sundaram KR et al (2016) A randomized study of coconut oil versus sunflower oil on cardiovascular risk factors in patients with stable coronary heart disease. Indian Heart J 68:498–506. https://doi.org/10.1016/j.ihj.2015.10.384
Matualatupauw JC, Bohl M, Gregersen S et al (2017) Dietary medium-chain saturated fatty acids induce gene expression of energy metabolism-related pathways in adipose tissue of abdominally obese subjects. Int J Obes (Lond) 41:1348–1354. https://doi.org/10.1038/ijo.2017.120
Saraswathi V, Kumar N, Gopal T et al (2020) Lauric acid versus palmitic acid: effects on adipose tissue inflammation, insulin resistance, and non-alcoholic fatty liver disease in obesity. Biology (Basel) 9:346. https://doi.org/10.3390/biology9110346
Acknowledgements
The authors acknowledge Dylbert Fragoso for technical assistance in the English language.
Funding
This work was supported by “Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq” through fellowships (305725/2020-3; 164013/2020-2).
Author information
Authors and Affiliations
Contributions
GJ conducted the literature search conceptualized and executed the review and drafted the first manuscript. LPP guided the development of the work. All the authors approved the final version of the manuscript and takes responsibility for all aspects of the reliability, freedom from bias, and interpretation of the data presented.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Consent for publication
All the authors agree with this publication.
Ethical approval and consent to participate
None to disclosure.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jamar, G., Pisani, L.P. Inflammatory crosstalk between saturated fatty acids and gut microbiota–white adipose tissue axis. Eur J Nutr 62, 1077–1091 (2023). https://doi.org/10.1007/s00394-022-03062-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-022-03062-z