Abstract
Purpose
To assess whether polymorphisms of haptoglobin (Hp) modify the relationship between dietary iron and the risk of gestational iron-deficiency anemia (IDA).
Methods
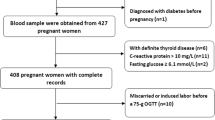
This study analyzed 1430 singleton pregnant women aged 20 ~ ≤ 48 years from the 2017–2019 National Nutrition and Health Survey of Pregnant Women in Taiwan. Sociodemographic, blood biochemical, Hp phenotype, and 24-h dietary recall data were collected. Erythropoiesis-related total prenatal supplementation was defined as the reported use of multivitamins and minerals, vitamin B complex, folate, and iron.
Results
Distributions of the Hp 1-1, Hp 2-1, and Hp 2-2 phenotypes were 13.6, 39.8, and 46.5%, respectively. Women with the Hp 1-1 phenotype had the lowest mean levels of serum ferritin (p-trend = 0.017), the highest prevalence of gestational ID (p-trend = 0.033) as well as the highest prevalence of gestational IDA (did not reach statistical differences, p-trend = 0.086). A gene–diet interaction on serum ferritin was observed between the Hp 1 and Hp 2 (2-1/2-2) alleles (p < 0.001). An adjusted multivariate logistic regression showed that compared to those with a normal blood iron status and who reported using erythropoiesis-related total prenatal supplements, those who did not had a 4.05-fold [odds ratio (OR) = 4.05 (95% confidence interval (CI) 2.63–6.24), p < 0.001] increased risk of gestational IDA. The corresponding ORs for carriers of the Hp 1 and Hp 2 alleles were 4.78 (95% CI 1.43–15.99) and 3.79 (95% CI 2.37–6.06), respectively.
Conclusion
Pregnant women who are Hp 1 carriers are at increased risk for developing IDA if they do not meet the recommended dietary allowance for iron or use erythropoiesis-related prenatal supplements.

Similar content being viewed by others
Availability of data and materials
Data described in the manuscript, code book, and analytic code will be made available from the corresponding author on reasonable request.
Abbreviations
- ANOVA:
-
Analysis of variance
- CD163:
-
Cluster of differentiation 163
- DRI:
-
Dietary reference intake
- Hb:
-
Hemoglobin
- Hp:
-
Haptoglobin
- ID:
-
Iron deficiency
- IDA:
-
Iron-deficiency anemia
- NAHSIT:
-
Nutrition and Health Survey in Taiwan
- NTD:
-
New Taiwan Dollar
- pBMI:
-
Pre-pregnancy body mass index
- pBW:
-
Pre-pregnancy body weight
- RDAs:
-
Recommended Dietary Allowances
- SD:
-
Standard deviation
- SF:
-
Serum ferritin
- TS:
-
Transferrin saturation
References
Teichman J, Nisenbaum R, Lausman A, Sholzberg M (2021) Suboptimal iron deficiency screening in pregnancy and the impact of socioeconomic status in a high-resource setting. Blood Adv 5(22):4666–4673. https://doi.org/10.1182/bloodadvances.2021004352
Fisher AL, Nemeth E (2017) Iron homeostasis during pregnancy. Am J Clin Nutr 106(Suppl 6):1567S-1574S. https://doi.org/10.3945/ajcn.117.155812
Sangkhae V, Fisher AL, Wong S, Koenig MD, Tussing-Humphreys L, Chu A, Lelić M, Ganz T, Nemeth E (2021) Effects of maternal iron status on placental and fetal iron homeostasis. J Clin Investig. https://doi.org/10.1172/JCI127341
Stoffel NU, Zimmermann MB, Cepeda-Lopez AC, Cervantes-Gracia K, Llanas-Cornejo D, Zeder C, Tuntipopipat S, Moungmaithong S, Densupsoontorn N, Quack-Loetscher K, Gowachirapant S, Herter-Aeberli I (2021) Maternal iron kinetics and maternal-fetal iron transfer in normal weight and overweight pregnancy. Am J Clin Nutr. https://doi.org/10.1093/ajcn/nqab406
World Health Organization (2015) The global prevalence of anaemia in 2011. WHO Document Production Services, Geneva
World Health Organization (2016) WHO recommendations on antenatal care for a positive pregnancy experience. WHO Document Production Services, Geneva
Milman N, Taylor CL, Merkel J, Brannon PM (2017) Iron status in pregnant women and women of reproductive age in Europe. Am J Clin Nutr 106(suppl 6):1655S-1662S. https://doi.org/10.3945/ajcn.117.156000
Dewey KG, Oaks BM (2017) U-shaped curve for risk associated with maternal hemoglobin, iron status, or iron supplementation. Am J Clin Nutr 106(Suppl 6):1694s–1702s. https://doi.org/10.3945/ajcn.117.156075
Ordovas JM, Ferguson LR, Tai ES, Mathers JC (2018) Personalised nutrition and health. BMJ (Clinical Research ed) 361:bmj2173. https://doi.org/10.1136/bmj.k2173
Cahill LE, Rimm EB (2015) Diet-gene interactions: haptoglobin genotype and nutrient status. In: Bendich A, Deckelbaum RJ (eds) Preventive nutrition: the comprehensive guide for health professionals. Springer, Cham, pp 115–129
Andersen CBF, Stodkilde K, Saederup KL, Kuhlee A, Raunser S, Graversen JH, Moestrup SK (2017) Haptoglobin. Antioxid Redox Signal 26(14):814–831. https://doi.org/10.1089/ars.2016.6793
Langlois MR, Delanghe JR (1996) Biological and clinical significance of haptoglobin polymorphism in humans. Clin Chem 42(10):1589–1600
Delanghe JR, Langlois MR (2002) Haptoglobin polymorphism and body iron stores. Clin Chem Lab Med 40(3):212–216. https://doi.org/10.1515/CCLM.2002.035
Langlois MR, Martin ME, Boelaert JR, Beaumont C, Taes YE, De Buyzere ML, Bernard DR, Neels HM, Delanghe JR (2000) The haptoglobin 2–2 phenotype affects serum markers of iron status in healthy males. Clin Chem 46(10):1619–1625
Nyakeriga AM, Troye-Blomberg M (2013) Haptoglobin phenotypes and iron status in children living in a malaria endemic area of Kenyan coast. Acta Trop 126(2):127–131. https://doi.org/10.1016/j.actatropica.2013.02.004
Kasvosve I, Gordeuk VR, Delanghe JR, Gomo ZA, Gangaidzo IT, Khumalo H, Moyo VM, Saungweme T, Mvundura E, Boelaert JR (2002) Iron status in black persons is not influenced by haptoglobin polymorphism. Clin Chem Lab Med 40(8):810–813. https://doi.org/10.1515/CCLM.2002.140
Cahill LE, El-Sohemy A (2010) Haptoglobin genotype modifies the association between dietary vitamin C and serum ascorbic acid deficiency. Am J Clin Nutr 92(6):1494–1500. https://doi.org/10.3945/ajcn.2010.29306
Tang KY, Huang SY, Cheng TM, Bai CH, Chang JS (2020) Haptoglobin phenotype influences the effectiveness of diet-induced weight loss in middle-age abdominally obese women with metabolic abnormalities. Clin Nutr 39(1):225–233. https://doi.org/10.1016/j.clnu.2019.01.019
Tu SH, Chen C, Hsieh YT, Chang HY, Yeh CJ, Lin YC, Pan WH (2011) Design and sample characteristics of the 2005–2008 Nutrition and Health Survey in Taiwan. Asia Pac J Clin Nutr 20(2):225–237
Hughes RA, Heron J, Sterne JAC, Tilling K (2019) Accounting for missing data in statistical analyses: multiple imputation is not always the answer. Int J Epidemiol 48(4):1294–1304. https://doi.org/10.1093/ije/dyz032
Taiwan Food and Drug Administration Ministry of Health and Welfare (2012) 7th Edition of the Taiwan's Dietary Reference Intakes. Taiwan Food and Drug Administration Ministry of Health and Welfare, Taipei
Health Promotion Administration Ministry of Health and Welfare (2018) Maternal health booklet. Health Promotion Administration Ministry of Health and Welfare, Taipei
World Health Organization (2001) Iron deficiency anaemia: assessment, prevention and control: a guide for programme managers. World Health Organization, Geneva
Centers for Disease Control and Prevention (1998) Recommendations to prevent and control iron deficiency in the United States. MMWR Recomm Rep 47(RR-3):1–29
Chen KJ, Pan WH, Lin YC, Lin BF (2011) Trends in folate status in the Taiwanese population aged 19 years and older from the Nutrition and Health Survey in Taiwan 1993–1996 to 2005–2008. Asia Pac J Clin Nutr 20(2):275–282
de Benoist B (2008) Conclusions of a WHO technical consultation on folate and vitamin B12 deficiencies. Food Nutr Bull 29(2 Suppl):S238-244. https://doi.org/10.1177/15648265080292S129
Kristiansen M, Graversen JH, Jacobsen C, Sonne O, Hoffman HJ, Law SK, Moestrup SK (2001) Identification of the haemoglobin scavenger receptor. Nature 409(6817):198–201. https://doi.org/10.1038/35051594
Slusarczyk P, Mleczko-Sanecka K (2021) The multiple facets of iron recycling. Genes 12(9):1364. https://doi.org/10.3390/genes12091364
Chang TY, Liu KL, Chang CS, Su CT, Chen SH, Lee YC, Chang JS (2018) Ferric citrate supplementation reduces red-blood-cell aggregation and improves CD163+ macrophage-mediated hemoglobin metabolism in a rat model of high-fat-diet-induced obesity. Mol Nutr Food Res. https://doi.org/10.1002/mnfr.201700442
Semba RD, Bloem MW (2002) The anemia of vitamin A deficiency: epidemiology and pathogenesis. Eur J Clin Nutr 56(4):271–281. https://doi.org/10.1038/sj.ejcn.1601320
Dreyfuss ML, Stoltzfus RJ, Shrestha JB, Pradhan EK, LeClerq SC, Khatry SK, Shrestha SR, Katz J, Albonico M, West KP Jr (2000) Hookworms, malaria and vitamin A deficiency contribute to anemia and iron deficiency among pregnant women in the plains of Nepal. J Nutr 130(10):2527–2536. https://doi.org/10.1093/jn/130.10.2527
da Cunha MSB, Campos Hankins NA, Arruda SF (2019) Effect of vitamin A supplementation on iron status in humans: A systematic review and meta-analysis. Crit Rev Food Sci Nutr 59(11):1767–1781. https://doi.org/10.1080/10408398.2018.1427552
Mendes JF, Siqueira EM, de Brito ESJG, Arruda SF (2016) Vitamin A deficiency modulates iron metabolism independent of hemojuvelin (Hfe2) and bone morphogenetic protein 6 (Bmp6) transcript levels. Genes Nutr 11:1. https://doi.org/10.1186/s12263-016-0519-4
Paciolla C, Fortunato S, Dipierro N, Paradiso A, De Leonardis S, Mastropasqua L, de Pinto MC (2019) Vitamin C in plants: from functions to biofortification. Antioxidants (Basel). https://doi.org/10.3390/antiox8110519
Powers JM, Auerbach M (2020) Iron supplementation in infants: a reflection on hepcidin and fractional iron absorption. Am J Clin Nutr 112(4):909–910. https://doi.org/10.1093/ajcn/nqaa224
Milman NT (2021) Managing genetic hemochromatosis: an overview of dietary measures, which may reduce intestinal iron absorption in persons with iron overload. Gastroenterol Res 14(2):66–80. https://doi.org/10.14740/gr1366
Wobeto VPdA, Zaccariotto TR, Sonati MdF (2008) Polymorphism of human haptoglobin and its clinical importance. Genet Mol Biol 31(3):602–620
Zhao H, Zhang G, Duan Y, Yu S (1993) Haptoglobin types in Chinese ethnic groups. Hum Hered 43(2):131–133. https://doi.org/10.1159/000154130
Asleh R, Briasoulis A, Berinstein EM, Wiener JB, Palla M, Kushwaha SS, Levy AP (2018) Meta-analysis of the association of the haptoglobin genotype with cardiovascular outcomes and the pharmacogenomic interactions with vitamin E supplementation. Pharmacogenom Personal Med 11:71–82. https://doi.org/10.2147/PGPM.S159454
Mayasari NR, Hu TY, Chao JC, Bai CH, Chen YC, Huang YL, Chang CC, Wang FF, Hadi H, Nurwanti E, Chang JS (2021) Associations of the pre-pregnancy weight status with anaemia and the erythropoiesis-related micronutrient status. Public Health Nutr 24(18):6247–6257. https://doi.org/10.1017/s1368980021002627
Acknowledgements
All the authors would like to thank the Ministry of Health and Welfare, Taiwan for allowing us to retrieve data of the NAHSIT-PW 2017–2019.
Funding
Dr. Jung-Su Chang was supported by grants from Taipei Medical University Hospital [111TMU-TMUH-054] and the Ministry of Science and Technology, Taiwan [MOST109-2923-B-038-001-MY3 and MOST 111-2320-B-038-030-MY3].
Author information
Authors and Affiliations
Contributions
JSC conceived and designed the research; TYH, CHB, and JCC conducted the research; TMC, YLH, and FFW provided the materials; TYH and NRM performed the statistical analyses; TYH, NRM, and JSC wrote the manuscript; JSC had primary responsibility for the final content; and all the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethics approval
This study was approved by the Taipei Medical University Institutional Review Board (TMU-JIRB N201707039) (https://ohr.tmu.edu.tw).
Supplementary Information
Below is the link to the electronic supplementary material.
Fig S1.
Flow chart of the study participants
Supplementary file1 (DOCX 1155 KB)
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hu, TY., Mayasari, N.R., Cheng, TM. et al. Polymorphisms of haptoglobin modify the relationship between dietary iron and the risk of gestational iron-deficiency anemia. Eur J Nutr 62, 299–309 (2023). https://doi.org/10.1007/s00394-022-02987-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-022-02987-9