Abstract
Purpose
The present study investigated whether maternal curcumin supplementation might protect against intra-uterine growth retardation (IUGR) induced intestinal damage and modulate gut microbiota in male mice offspring.
Methods
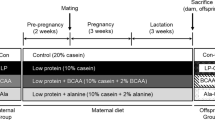
In total, 36 C57BL/6 mice (24 females and 12 males, 6–8 weeks old) were randomly divided into three groups based on the diet before and throughout pregnancy and lactation: (1) normal protein (19%), (2) low protein (8%), and (3) low protein (8%) + 600 mg kg−1 curcumin. Offspring were administered a control diet until postnatal day 35.
Results
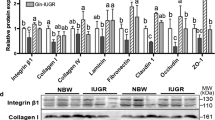
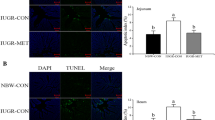
Maternal curcumin supplementation could normalize the maternal protein deficiency-induced decrease in jejunal SOD activity (NP = 200.40 ± 10.58 U/mg protein; LP = 153.30 ± 5.51 U/mg protein; LPC = 185.40 ± 9.52 U/mg protein; P < 0.05) and T-AOC content (NP = 138.90 ± 17.51 U/mg protein; LP = 84.53 ± 5.42 U/mg protein; LPC = 99.73 ± 12.88 U/mg protein; P < 0.05) in the mice offspring. Maternal curcumin supplementation increased the maternal low protein diet-induced decline in the ratio of villus height-to-crypt depth (NP = 2.23 ± 0.19; LP = 1.90 ± 0.06; LPC = 2.56 ± 0.20; P < 0.05), the number of goblet cells (NP = 12.72 ± 1.16; LP = 7.04 ± 0.53; LPC = 13.10 ± 1.17; P < 0.05), and the ratio of PCNA-positive cells (NP = 13.59 ± 1.13%; LP = 2.42 ± 0.74%; LPC = 6.90 ± 0.96%; P < 0.05). It also reversed the maternal protein deficiency-induced increase of the body weight (NP = 13.00 ± 0.48 g; LP = 16.49 ± 0.75 g; LPC = 10.65 ± 1.12 g; P < 0.05), the serum glucose levels (NP = 5.32 ± 0.28 mmol/L; LP = 6.82 ± 0.33 mmol/L; LPC = 4.69 ± 0.35 mmol/L; P < 0.05), and the jejunal apoptotic index (NP = 6.50 ± 1.58%; LP = 10.65 ± 0.75%; LPC = 5.24 ± 0.71%; P < 0.05). Additionally, maternal curcumin supplementation enhanced the gene expression level of Nrf2 (NP = 1.00 ± 0.12; LP = 0.73 ± 0.10; LPC = 1.34 ± 0.12; P < 0.05), Sod2 (NP = 1.00 ± 0.04; LP = 0.85 ± 0.04; LPC = 1.04 ± 0.04; P < 0.05) and Ocln (NP = 1.00 ± 0.09; LP = 0.94 ± 0.10; LPC = 1.47 ± 0.09; P < 0.05) in the jejunum. Furthermore, maternal curcumin supplementation normalized the relative abundance of Lactobacillus (NP = 31.56 ± 6.19%; LP = 7.60 ± 2.33%; LPC = 17.79 ± 2.41%; P < 0.05) and Desulfovibrio (NP = 3.63 ± 0.93%; LP = 20.73 ± 3.96%; LPC = 13.96 ± 4.23%; P < 0.05), and the ratio of Firmicutes/Bacteroidota (NP = 2.84 ± 0.64; LP = 1.21 ± 0.30; LPC = 1.79 ± 0.15; P < 0.05). Moreover, Lactobacillus was positively correlated with the SOD activity, and it was negatively correlated with Il − 1β expression (P < 0.05). Desulfovibrio was negatively correlated with the SOD activity and the jejunal expression of Sod1, Bcl − 2, Card11, and Zo − 1 (P < 0.05).
Conclusions
Maternal curcumin supplementation could improve intestinal integrity, oxidative status, and gut microbiota in male mice offspring with IUGR.









Similar content being viewed by others
References
Longo S, Borghesi A, Tzialla C, Stronati M (2014) IUGR and infections. Early Hum Dev 90(Suppl 1):S42-44. https://doi.org/10.1016/s0378-3782(14)70014-3
Darendeliler F (2019) IUGR: genetic influences, metabolic problems, environmental associations/triggers, current and future management. Best Pract Res Clin Endocrinol Metab 33(3):101260. https://doi.org/10.1016/j.beem.2019.01.001
Wang C, Zhang RM, Zhou L, He J, Huang Q, Siyal FA, Zhang L, Zhong X, Wang T (2017) Intrauterine growth retardation promotes fetal intestinal autophagy in rats via the mechanistic target of rapamycin pathway. J Reprod Dev 63(6):547–554. https://doi.org/10.1262/jrd.2017-050
Bozzetti V, Paterlini G, Bel F, Visser GH, Tosetti L, Gazzolo D, Tagliabue PE (2016) Cerebral and somatic NIRS-determined oxygenation in IUGR preterm infants during transition. J Matern Fetal Neonatal Med 29(3):443–446. https://doi.org/10.3109/14767058.2014.1003539
Zhang W, Ma C, Xie P, Zhu Q, Wang X, Yin Y, Kong X (2019) Gut microbiota of newborn piglets with intrauterine growth restriction have lower diversity and different taxonomic abundances. J Appl Microbiol 127(2):354–369. https://doi.org/10.1111/jam.14304
Adak A, Khan MR (2019) An insight into gut microbiota and its functionalities. Cell Mol Life Sci 76(3):473–493. https://doi.org/10.1007/s00018-018-2943-4
Anand P, Thomas SG, Kunnumakkara AB, Sundaram C, Harikumar KB, Sung B, Tharakan ST, Misra K, Priyadarsini IK, Rajasekharan KN, Aggarwal BB (2008) Biological activities of curcumin and its analogues (Congeners) made by man and mother nature. Biochem Pharmacol 76(11):1590–1611. https://doi.org/10.1016/j.bcp.2008.08.008
Samarghandian S, Azimi-Nezhad M, Farkhondeh T, Samini F (2017) Anti-oxidative effects of curcumin on immobilization-induced oxidative stress in rat brain, liver and kidney. Biomed Pharmacother 87:223–229. https://doi.org/10.1016/j.biopha.2016.12.105
Ruan D, Wang WC, Lin CX, Fouad AM, Chen W, Xia WG, Wang S, Luo X, Zhang WH, Yan SJ, Zheng CT, Yang L (2019) Effects of curcumin on performance, antioxidation, intestinal barrier and mitochondrial function in ducks fed corn contaminated with ochratoxin A. Animal 13(1):42–52. https://doi.org/10.1017/s1751731118000678
Kanter M, Takir M, Mutlu HH, Kanter B, Kostek O, Toprak AE (2016) Protective effects of curcumin on intestinal damage in cholestatic rats. J Invest Surg 29(3):128–136. https://doi.org/10.3109/08941939.2015.1088604
Feng W, Wang H, Zhang P, Gao C, Tao J, Ge Z, Zhu D, Bi Y (2017) Modulation of gut microbiota contributes to curcumin-mediated attenuation of hepatic steatosis in rats. Biochim Biophys Acta Gen Subj 1861(7):1801–1812. https://doi.org/10.1016/j.bbagen.2017.03.017
Shen L, Liu L, Ji HF (2017) Regulative effects of curcumin spice administration on gut microbiota and its pharmacological implications. Food Nutr Res 61(1):1361780. https://doi.org/10.1080/16546628.2017.1361780
Qi LN, Jiang JL, Zhang JF, Zhang LL, Wang T (2020) Maternal curcumin supplementation ameliorates placental function and fetal growth in mice with intrauterine growth retardation. Biol Reprod 102(5):1090–1101. https://doi.org/10.1093/biolre/ioaa005
Chisaka T, Mogi M, Nakaoka H, Kan-No H, Tsukuda K, Wang XL, Bai HY, Shan BS, Kukida M, Iwanami J, Higaki T, Ishii E, Horiuchi M (2016) Low-protein diet-induced fetal growth restriction leads to exaggerated proliferative response to vascular injury in postnatal life. Am J Hypertens 29(1):54–62. https://doi.org/10.1093/ajh/hpv072
Bhasin KK, van Nas A, Martin LJ, Davis RC, Devaskar SU, Lusis AJ (2009) Maternal low-protein diet or hypercholesterolemia reduces circulating essential amino acids and leads to intrauterine growth restriction. Diabetes 58(3):559–566. https://doi.org/10.2337/db07-1530
Yu M, Tan L, Chen J, Zhai Y, Wu X, Xu H, Shen Q (2019) Intrauterine low-protein diet disturbs metanephric gene expression and induces urinary tract developmental abnormalities in mice. Biochem Biophys Res Commun 513(3):732–739. https://doi.org/10.1016/j.bbrc.2019.04.057
Zhang JF, Bai KW, He JT, Niu Y, Lu Y, Zhang L, Wang T (2018) Curcumin attenuates hepatic mitochondrial dysfunction through the maintenance of thiol pool, inhibition of mtDNA damage, and stimulation of the mitochondrial thioredoxin system in heat-stressed broilers. J Anim Sci 96(3):867–879. https://doi.org/10.1093/jas/sky009
Afrin R, Arumugam S, Rahman A, Wahed MI, Karuppagounder V, Harima M, Suzuki H, Miyashita S, Suzuki K, Yoneyama H, Ueno K, Watanabe K (2017) Curcumin ameliorates liver damage and progression of NASH in NASH-HCC mouse model possibly by modulating HMGB1-NF-κB translocation. Int Immunopharmacol 44:174–182. https://doi.org/10.1016/j.intimp.2017.01.016
Jiang J, Qi L, Wei Q, Shi F (2021) Maternal stevioside supplementation ameliorates intestinal mucosal damage and modulates gut microbiota in chicken offspring challenged with lipopolysaccharide. Food Funct. https://doi.org/10.1039/d0fo02871a
Jiang JL, Qi LN, Lv ZP, Wei QW, Shi FX (2021) Dietary stevioside supplementation increases feed intake by altering the hypothalamic transcriptome profile and gut microbiota in broiler chickens. J Sci Food Agric 101(5):2156–2167. https://doi.org/10.1002/jsfa.10838
Ye L, Zhang Q, Xin F, Cao B, Qian L, Dong Y (2021) Neonatal milk fat globule membrane supplementation during breastfeeding ameliorates the deleterious effects of maternal high-fat diet on metabolism and modulates gut microbiota in adult mice offspring in a sex-specific way. Front Cell Infect Microbiol 11:621957. https://doi.org/10.3389/fcimb.2021.621957
Wankhade UD, Zhong Y, Kang P, Alfaro M, Chintapalli SV, Piccolo BD, Mercer KE, Andres A, Thakali KM, Shankar K (2018) Maternal high-fat diet programs offspring liver steatosis in a sexually dimorphic manner in association with changes in gut microbial ecology in mice. Sci Rep 8(1):16502. https://doi.org/10.1038/s41598-018-34453-0
Li C, Xu JJ, Hu HT, Shi CY, Yu CJ, Sheng JZ, Wu YT, Huang HF (2021) Amylin receptor insensitivity impairs hypothalamic POMC neuron differentiation in the male offspring of maternal high-fat diet-fed mice. Mol Metab 44:101135. https://doi.org/10.1016/j.molmet.2020.101135
Xie S, Zhao SY, Jiang L, Lu L, Yang Q, Yu Q (2019) Lactobacillus reuteri stimulates intestinal epithelial proliferation and induces differentiation into goblet cells in young chickens. J Agric Food Chem 67(49):13758–13766. https://doi.org/10.1021/acs.jafc.9b06256
Hall PA, Levison DA, Woods AL, Yu CC, Kellock DB, Watkins JA, Barnes DM, Gillett CE, Camplejohn R, Dover R et al (1990) Proliferating cell nuclear antigen (PCNA) immunolocalization in paraffin sections: an index of cell proliferation with evidence of deregulated expression in some neoplasms. J Pathol 162(4):285–294. https://doi.org/10.1002/path.1711620403
Günther C, Neumann H, Neurath MF, Becker C (2013) Apoptosis, necrosis and necroptosis: cell death regulation in the intestinal epithelium. Gut 62(7):1062–1071. https://doi.org/10.1136/gutjnl-2011-301364
Wu G, Bazer FW, Wallace JM, Spencer TE (2006) Board-invited review: intrauterine growth retardation: implications for the animal sciences. J Anim Sci 84(9):2316–2337. https://doi.org/10.2527/jas.2006-156
Sutton GM, Centanni AV, Butler AA (2010) Protein malnutrition during pregnancy in C57BL/6J mice results in offspring with altered circadian physiology before obesity. Endocrinology 151(4):1570–1580. https://doi.org/10.1210/en.2009-1133
Souza Dde F, Ignácio-Souza LM, Reis SR, Reis MA, Stoppiglia LF, Carneiro EM, Boschero AC, Arantes VC, Latorraca MQ (2012) A low-protein diet during pregnancy alters glucose metabolism and insulin secretion. Cell Biochem Funct 30(2):114–121. https://doi.org/10.1002/cbf.1824
Zheng J, Xiao XH, Zhang Q, Wang T, Yu M, Xu J (2017) Maternal low-protein diet modulates glucose metabolism and hepatic MicroRNAs expression in the early life of offspring. Nutrients. https://doi.org/10.3390/nu9030205
Sellayah D, Cagampang FR (2018) The divergent effect of maternal protein restriction during pregnancy and postweaning high-fat diet feeding on blood pressure and adiposity in adult mouse offspring. Nutrients. https://doi.org/10.3390/nu10121832
Zou J, Zhang SS, Li PY, Zheng X, Feng D (2018) Supplementation with curcumin inhibits intestinal cholesterol absorption and prevents atherosclerosis in high-fat diet-fed apolipoprotein E knockout mice. Nutr Res 56:32–40. https://doi.org/10.1016/j.nutres.2018.04.017
Manzoni AG, Passos DF, da Silva JLG, Bernardes VM, Bremm JM, Jantsch MH, de Oliveira JS, Mann TR, de Andrade CM, Leal DBR (2019) Rutin and curcumin reduce inflammation, triglyceride levels and ADA activity in serum and immune cells in a model of hyperlipidemia. Blood Cells Mol Dis 76:13–21. https://doi.org/10.1016/j.bcmd.2018.12.005
Darmon N, Pélissier MA, Heyman M, Albrecht R, Desjeux JF (1993) Oxidative stress may contribute to the intestinal dysfunction of weanling rats fed a low protein diet. J Nutr 123(6):1068–1075. https://doi.org/10.1093/jn/123.6.1068
Saha SK, Lee SB, Won J, Choi HY, Kim K, Yang GM, Dayem AA, Cho SG (2017) Correlation between oxidative stress, nutrition, and cancer initiation. Int J Mol Sci. https://doi.org/10.3390/ijms18071544
Xue YF, Guo CZ, Hu F, Zhu W, Mao S (2020) Undernutrition-induced lipid metabolism disorder triggers oxidative stress in maternal and fetal livers using a model of pregnant sheep. FASEB J 34(5):6508–6520. https://doi.org/10.1096/fj.201902537R
Wu J, Ibtisham F, Niu YF, Wang Z, Li GH, Zhao Y, Nawab A, Xiao M, An L (2019) Curcumin inhibits heat-induced oxidative stress by activating the MAPK-Nrf2/ARE signaling pathway in chicken fibroblasts cells. J Therm Biol 79:112–119. https://doi.org/10.1016/j.jtherbio.2018.12.004
Ghiselli A, Serafini M, Natella F, Scaccini C (2000) Total antioxidant capacity as a tool to assess redox status: critical view and experimental data. Free Radic Biol Med 29(11):1106–1114. https://doi.org/10.1016/s0891-5849(00)00394-4
Nascimento L, Freitas CM, Silva-Filho R, Leite AC, Silva AB, da Silva AI, Ferreira DS, Pedroza AA, Maia MB, Fernandes MP, Lagranha C (2014) The effect of maternal low-protein diet on the heart of adult offspring: role of mitochondria and oxidative stress. Appl Physiol Nutr Metab 39(8):880–887. https://doi.org/10.1139/apnm-2013-0452
Ruan D, Zhu YW, Fouad AM, Yan SJ, Chen W, Zhang YN, Xia WG, Wang S, Jiang SQ, Yang L, Zheng CT (2019) Dietary curcumin enhances intestinal antioxidant capacity in ducklings via altering gene expression of antioxidant and key detoxification enzymes. Poult Sci 98(9):3705–3714. https://doi.org/10.3382/ps/pez058
Jambunathan LR, Neuhoff D, Younoszai MK (1981) Intestinal disaccharidases in malnourished infant rats. Am J Clin Nutr 34(9):1879–1884. https://doi.org/10.1093/ajcn/34.9.1879
Hampson DJ (1986) Alterations in piglet small intestinal structure at weaning. Res Vet Sci 40(1):32–40
Ducatelle R, Goossens E, De Meyer F, Eeckhaut V, Antonissen G, Haesebrouck F, Van Immerseel F (2018) Biomarkers for monitoring intestinal health in poultry: present status and future perspectives. Vet Res 49(1):43. https://doi.org/10.1186/s13567-018-0538-6
Firmansyah A, Suwandito L, Penn D, Lebenthal E (1989) Biochemical and morphological changes in the digestive tract of rats after prenatal and postnatal malnutrition. Am J Clin Nutr 50(2):261–268. https://doi.org/10.1093/ajcn/50.2.261
Singh V, Gowda CP, Singh V, Ganapathy AS, Karamchandani DM, Eshelman MA, Yochum GS, Nighot P, Spiegelman VS (2020) The mRNA-binding protein IGF2BP1 maintains intestinal barrier function by up-regulating occludin expression. J Biol Chem 295(25):8602–8612. https://doi.org/10.1074/jbc.AC120.013646
Shao YX, Lei Z, Wolf PG, Gao Y, Guo YM, Zhang BK (2017) Zinc supplementation, via GPR39, Upregulates PKCζ to protect intestinal barrier integrity in Caco-2 cells challenged by salmonella enterica serovar typhimurium. J Nutr 147(7):1282–1289. https://doi.org/10.3945/jn.116.243238
Birchenough GM, Johansson ME, Gustafsson JK, Bergström JH, Hansson GC (2015) New developments in goblet cell mucus secretion and function. Mucosal Immunol 8(4):712–719. https://doi.org/10.1038/mi.2015.32
Désir-Vigné A, Haure-Mirande V, de Coppet P, Darmaun D, Le Dréan G, Segain JP (2018) Perinatal supplementation of 4-phenylbutyrate and glutamine attenuates endoplasmic reticulum stress and improves colonic epithelial barrier function in rats born with intrauterine growth restriction. J Nutr Biochem 55:104–112. https://doi.org/10.1016/j.jnutbio.2017.12.007
Goldsworthy TL, Butterworth BE, Maronpot RR (1993) Concepts, labeling procedures, and design of cell proliferation studies relating to carcinogenesis. Environ Health Perspect 101(Suppl 5):59–65. https://doi.org/10.1289/ehp.93101s559
Tsai SH, Hsu LA, Tsai HY, Yeh YH, Lu CY, Chen PC, Wang JC, Chiu YL, Lin CY, Hsu YJ (2020) Aldehyde dehydrogenase 2 protects against abdominal aortic aneurysm formation by reducing reactive oxygen species, vascular inflammation, and apoptosis of vascular smooth muscle cells. FASEB J 34(7):9498–9511. https://doi.org/10.1096/fj.201902550RRR
Lee DE, Lee SJ, Kim SJ, Lee HS, Kwon OS (2019) Curcumin ameliorates nonalcoholic fatty liver disease through inhibition of O-GlcNAcylation. Nutrients. https://doi.org/10.3390/nu11112702
Clemente JC, Ursell LK, Parfrey LW, Knight R (2012) The impact of the gut microbiota on human health: an integrative view. Cell 148(6):1258–1270. https://doi.org/10.1016/j.cell.2012.01.035
Huang MK, Choi YJ, Houde R, Lee JW, Lee B, Zhao X (2004) Effects of Lactobacilli and an acidophilic fungus on the production performance and immune responses in broiler chickens. Poult Sci 83(5):788–795. https://doi.org/10.1093/ps/83.5.788
Angelakis E (2017) Weight gain by gut microbiota manipulation in productive animals. Microb Pathog 106:162–170. https://doi.org/10.1016/j.micpath.2016.11.002
McFadden RM, Larmonier CB, Shehab KW, Midura-Kiela M, Ramalingam R, Harrison CA, Besselsen DG, Chase JH, Caporaso JG, Jobin C, Ghishan FK, Kiela PR (2015) The role of curcumin in modulating colonic microbiota during colitis and colon cancer prevention. Inflamm Bowel Dis 21(11):2483–2494. https://doi.org/10.1097/mib.0000000000000522
Qiao Y, Sun J, Ding Y, Le G, Shi Y (2013) Alterations of the gut microbiota in high-fat diet mice is strongly linked to oxidative stress. Appl Microbiol Biotechnol 97(4):1689–1697. https://doi.org/10.1007/s00253-012-4323-6
Chen M, Hui S, Lang H, Zhou M, Zhang Y, Kang C, Zeng X, Zhang Q, Yi L, Mi M (2019) SIRT3 deficiency promotes high-fat diet-induced nonalcoholic fatty liver disease in correlation with impaired intestinal permeability through gut microbial dysbiosis. Mol Nutr Food Res 63(4):e1800612. https://doi.org/10.1002/mnfr.201800612
Li S, You J, Wang Z, Liu Y, Wang B, Du M, Zou T (2021) Curcumin alleviates high-fat diet-induced hepatic steatosis and obesity in association with modulation of gut microbiota in mice. Food Res Int 143:110270. https://doi.org/10.1016/j.foodres.2021.110270
Stojanov S, Berlec A, Štrukelj B (2020) The Influence of probiotics on the Firmicutes/Bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease. Microorganisms. https://doi.org/10.3390/microorganisms8111715
Chen M, Hou P, Zhou M, Ren Q, Wang X, Huang L, Hui S, Yi L, Mi M (2020) Resveratrol attenuates high-fat diet-induced non-alcoholic steatohepatitis by maintaining gut barrier integrity and inhibiting gut inflammation through regulation of the endocannabinoid system. Clin Nutr 39(4):1264–1275. https://doi.org/10.1016/j.clnu.2019.05.020
Yang Y, Zhang Y, Xu Y, Luo T, Ge Y, Jiang Y, Shi Y, Sun J, Le G (2019) Dietary methionine restriction improves the gut microbiota and reduces intestinal permeability and inflammation in high-fat-fed mice. Food Funct 10(9):5952–5968. https://doi.org/10.1039/c9fo00766k
Terzo S, Mulè F, Caldara GF, Baldassano S, Puleio R, Vitale M, Cassata G, Ferrantelli V, Amato A (2020) Pistachio consumption alleviates inflammation and improves gut microbiota composition in mice fed a high-fat diet. Int J Mol Sci. https://doi.org/10.3390/ijms21010365
Li Y (2018) Epigenetic mechanisms link maternal diets and gut microbiome to obesity in the offspring. Front Genet 9:342. https://doi.org/10.3389/fgene.2018.00342
Li Y, Tollefsbol TO (2010) Impact on DNA methylation in cancer prevention and therapy by bioactive dietary components. Curr Med Chem 17(20):2141–2151. https://doi.org/10.2174/092986710791299966
Fu Q, McKnight RA, Yu X, Wang L, Callaway CW, Lane RH (2004) Uteroplacental insufficiency induces site-specific changes in histone H3 covalent modifications and affects DNA-histone H3 positioning in day 0 IUGR rat liver. Physiol Genomics 20(1):108–116. https://doi.org/10.1152/physiolgenomics.00175.2004
Zheng J, Wu C, Lin Z, Guo Y, Shi L, Dong P, Lu Z, Gao S, Liao Y, Chen B, Yu F (2014) Curcumin up-regulates phosphatase and tensin homologue deleted on chromosome 10 through microRNA-mediated control of DNA methylation–a novel mechanism suppressing liver fibrosis. FEBS J 281(1):88–103. https://doi.org/10.1111/febs.12574
Gan L, Li C, Wang J, Guo X (2016) Curcumin modulates the effect of histone modification on the expression of chemokines by type II alveolar epithelial cells in a rat COPD model. Int J Chron Obstruct Pulmon Dis 11:2765–2773. https://doi.org/10.2147/copd.s113978
Bhat MI, Kapila R (2017) Dietary metabolites derived from gut microbiota: critical modulators of epigenetic changes in mammals. Nutr Rev 75(5):374–389. https://doi.org/10.1093/nutrit/nux001
Ye J, Wu W, Li Y, Li L (2017) Influences of the gut microbiota on DNA methylation and histone modification. Dig Dis Sci 62(5):1155–1164. https://doi.org/10.1007/s10620-017-4538-6
Acknowledgements
This research was funded by The National Natural Science Foundation of China (No.31772634).
Author information
Authors and Affiliations
Contributions
LQ and TW: conceived and designed the experiment; LQ and JJ: contributed to bioinformatics and statistical analyses. LQ, JJ, JZ, LZ, and TW: conducted the experiment; LQ: written original draft; LQ, JJ, and TW: edited and reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All experiments were approved by the Animal Care and Use Committee of Nanjing Agricultural University (SYXK (SU) 2017-0007).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Qi, L., Jiang, J., Zhang, J. et al. Effect of maternal curcumin supplementation on intestinal damage and the gut microbiota in male mice offspring with intra-uterine growth retardation. Eur J Nutr 61, 1875–1892 (2022). https://doi.org/10.1007/s00394-021-02783-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-021-02783-x