Abstract
Purpose
Sulfur amino acid (SAA) consumption in Western countries is far greater than recommended levels. In preclinical studies, reduced SAA intake enhanced longevity and reduced risk for numerous chronic diseases. The current objective was to examine for associations between the intake of total SAA, including methionine (Met) and cysteine (Cys), and all-cause and disease-specific mortality US adults.
Methods
This prospective analysis included 15,083 US adult participants (mean age = 46.7 years) from the Third National Examination and Nutritional Health Survey (NHANES III, 1988–1994) with available mortality status (National Death Registry, 1988–2011). Dietary SAA intake was obtained from 24-h recall data. Associations between quintile (Q) of SAA intake (expressed as absolute intake or protein density) and mortality were assessed using Cox proportional hazard models and expressed as hazard ratio (HR).
Results
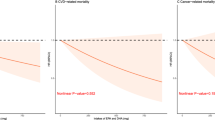
During follow-up (mean = 16.9 years), 4636 deaths occurred. After multivariable adjustment (including demographics and traditional risk factors, such as fat and other micronutrients intake), diabetes-caused mortality rates were nearly threefold higher in the highest compared to lowest SAA intake quintiles [HRQ5–Q1 total SAA, 2.68 (1.46–4.90); HRQ5–Q1 methionine, 2.45 (1.37–4.38); HRQ5–Q1 cysteine, 2.91 (1.57–5.37)] (P < 0.01)]. Higher total SAA protein density was also associated with diabetes-caused mortality [HRQ5–Q1 1.75 (1.31–2.35)]. Associations between SAA intake and all-cause mortality, and mortality caused by other major diseases were not detected.
Conclusion
Results suggest that high-SAA diets are associated with increased risk for diabetes mortality and that lowering intake towards to Recommended Dietary Allowance levels could lead to reductions in lifetime risk.

Similar content being viewed by others
Availability of data and material
Detailed NHANES survey descriptions, methodology, sampling procedures, laboratory test procedures, and data tables are publicly available (www.cdc.gov.nchs/nhanes/).
Code availability
Not aplicable.
References
Tesseraud S, Metayer Coustard S, Collin A, Seiliez I (2009) Role of sulfur amino acids in controlling nutrient metabolism and cell functions: implications for nutrition. Br J Nutr 101(8):1132–1139. https://doi.org/10.1017/S0007114508159025
Di Buono M, Wykes LJ, Ball RO, Pencharz PB (2001) Dietary cysteine reduces the methionine requirement in men. Am J Clin Nutr 74(6):761–766. https://doi.org/10.1093/ajcn/74.6.761
World Health Organization (2007) Protein and amino acid requirements in human nutrition. WHO technical report series 935
Institute of Medicine (2005) Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. The National Academies Press, Washington, DC. https://doi.org/10.17226/10490
Baker DH, Dilger RN (2009) Sulfur amino acid deficiency and toxicity: research with animal models. In: Glutathione and sulfur amino acids in human health and disease. Wiley, pp 289–316
Garlick PJ (2006) Toxicity of methionine in humans. J Nutr 136(6):1722S-1725S
Benevenga N, Steele R (1984) Adverse effects of excessive consumption of amino acids. Annu Rev Nutr 4(1):157–181
Pamplona R, Barja G (2006) Mitochondrial oxidative stress, aging and caloric restriction: the protein and methionine connection. Biochim Biophys Acta 1757(5–6):496–508
Benevenga NJ, Yeh MH, Lalich JJ (1976) Growth depression and tissue reaction to the consumption of excess dietary methionine and S-methyl-L-cysteine. J Nutr 106(12):1714–1720. https://doi.org/10.1093/jn/106.12.1715
Orentreich N, Matias JR, Defelice A, Zimmerman JA (1993) Low methionine ingestion by rats extends life-span. J Nutr 123(2):269–274 (<Go to ISI>://WOS:A1993KL61200013)
Zimmerman JA, Malloy V, Krajcik R, Orentreich N (2003) Nutritional control of aging. Exp Gerontol 38(1–2):47–52
Ables GP, Johnson JE (2017) Pleiotropic responses to methionine restriction. Exp Gerontol 94:83–88
Komninou D, Leutzinger Y, Reddy BS, Richie JP Jr (2006) Methionine restriction inhibits colon carcinogenesis. Nutr Cancer 54(2):202–208
Grant L, Lees EK, Forney LA, Mody N, Gettys T, Brown PA, Wilson HM, Delibegovic M (2016) Methionine restriction improves renal insulin signalling in aged kidneys. Mech Ageing Dev 157:35–43
Richie JP, Komninou D, Leutzinger Y, Kleinman W, Orentreich N, Malloy V, Zimmerman JA (2004) Tissue glutathione and cysteine levels in methionine-restricted rats. Nutrition 20(9):800–805
Richie JP Jr, Leutzinger Y, Parthasarathy S, Malloy V, Orentreich N, Zimmerman JA (1994) Methionine restriction increases blood glutathione and longevity in F344 rats. FASEB J 8(15):1302–1307. https://doi.org/10.1096/fasebj.8.15.8001743
Elshorbagy AK, Valdivia-Garcia M, Mattocks DA, Plummer JD, Smith AD, Drevon CA, Refsum H, Perrone CE (2011) Cysteine supplementation reverses methionine restriction effects on rat adiposity: significance of stearoyl-coenzyme A desaturase. J Lipid Res 52(1):104–112
Dong Z, Sinha R, Richie JP (2018) Disease prevention and delayed aging by dietary sulfur amino acid restriction: translational implications. Ann N Y Acad Sci 1418:44–55
Dong Z, Gao X, Chinchilli VM, Sinha R, Muscat J, Winkels RM, Richie JP Jr (2020) Association of sulfur amino acid consumption with cardiometabolic risk factors: Cross-sectional findings from NHANES III. EClinicalMedicine 19:100248. https://doi.org/10.1016/j.eclinm.2019.100248
US Department of Health & Human Services (2017) NCHS Research Ethics Review Board (ERB) Approval. https://www.cdc.gov/nchs/nhanes/irba98.htm. Accessed 1 June 2021
National Center for Health Statistics (1994) Plan and operation of the third National Health and Nutrition Examination Survey, 1988–94. Vital Health Stat 32:1–407
US Department of Health and Human Services (1996) NHANES Ill Reference manuals and Reports (CD-ROM). Centers for Disease Control and Prevention, Hyattsville
US Department of Health and Human Services (1996) Third national health and nutrition examination survey, 1988–1994. Centers for Disease Control and Prevention, Hyattsville
Zheng Y, Li Y, Qi Q, Hruby A, Manson JE, Willett WC, Wolpin BM, Hu FB, Qi L (2016) Cumulative consumption of branched-chain amino acids and incidence of type 2 diabetes. Int J Epidemiol 45(5):1482–1492. https://doi.org/10.1093/ije/dyw143
US Department of Agriculture Ag Data Commons. Food and Nutrient Database for Dietary Studies (FNDDS). https://data.nal.usda.gov/dataset/food-and-nutrient-database-dietary-studies-fndds. Accessed 1 June 2021
Willett WC, Howe GR, Kushi LH (1997) Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 65(4):1220S-1228S
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150(9):604–612
World Health Organization (2004) International statistical classification of diseases and related health problems, vol 1. World Health Organization, Geneva
Willett W, Stampfer MJ (1986) Total energy intake: implications for epidemiologic analyses. Am J Epidemiol 124(1):17–27
Parekh N, Lin Y, Craft LL, Vadiveloo M, Lu-Yao GL (2012) Longitudinal associations of leisure-time physical activity and cancer mortality in the Third National Health and Nutrition Examination Survey (1986–2006). J Obes 2012:518358. https://doi.org/10.1155/2012/518358
Song M, Fung TT, Hu FB, Willett WC, Longo VD, Chan AT, Giovannucci EL (2016) Association of animal and plant protein intake with all-cause and cause-specific mortality. JAMA Intern Med 176(10):1453–1463. https://doi.org/10.1001/jamainternmed.2016.4182
Levine ME, Suarez JA, Brandhorst S, Balasubramanian P, Cheng CW, Madia F, Fontana L, Mirisola MG, Guevara-Aguirre J, Wan J, Passarino G, Kennedy BK, Wei M, Cohen P, Crimmins EM, Longo VD (2014) Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab 19(3):407–417. https://doi.org/10.1016/j.cmet.2014.02.006
Kelemen LE, Kushi LH, Jacobs DR Jr, Cerhan JR (2005) Associations of dietary protein with disease and mortality in a prospective study of postmenopausal women. Am J Epidemiol 161(3):239–249. https://doi.org/10.1093/aje/kwi038
Ables GP, Perrone CE, Orentreich D, Orentreich N (2012) Methionine-restricted C57BL/6J mice are resistant to diet-induced obesity and insulin resistance but have low bone density. PLoS ONE 7(12):e51357
Stone KP, Wanders D, Orgeron M, Cortez CC, Gettys TW (2014) Mechanisms of increased in vivo insulin sensitivity by dietary methionine restriction in mice. Diabetes 63(11):3721–3733
Yin J, Ren W, Chen S, Li Y, Han H, Gao J, Liu G, Wu X, Li T, Woo Kim S (2018) Metabolic regulation of methionine restriction in diabetes. Mol Nutr Food Res 62(10):1700951
Orgeron ML, Stone KP, Wanders D, Cortez CC, Van NT, Gettys TW (2014) The impact of dietary methionine restriction on biomarkers of metabolic health. Prog Mol Biol Transl Sci 121:351
Plaisance EP, Greenway FL, Boudreau A, Hill KL, Johnson WD, Krajcik RA, Perrone CE, Orentreich N, Cefalu WT, Gettys TW (2011) Dietary methionine restriction increases fat oxidation in obese adults with metabolic syndrome. J Clin Endocrinol Metab 96(5):E836–E840
Mohorko N, Petelin A, Jurdana M, Biolo G, Jenko-Pražnikar Z (2015) Elevated serum levels of cysteine and tyrosine: early biomarkers in asymptomatic adults at increased risk of developing metabolic syndrome. BioMed Res Int 2015:1–14
Elshorbagy AK, Valdivia-Garcia M, Refsum H, Butte N (2012) The association of cysteine with obesity, inflammatory cytokines and insulin resistance in Hispanic children and adolescents. PLoS ONE 7(9):e44166
Yin J, Ren W, Chen S, Li Y, Han H, Gao J, Liu G, Wu X, Li T, Woo Kim S, Yin Y (2018) Metabolic regulation of methionine restriction in diabetes. Mol Nutr Food Res 62(10):1700951. https://doi.org/10.1002/mnfr.201700951
Castano-Martinez T, Schumacher F, Schumacher S, Kochlik B, Weber D, Grune T, Biemann R, McCann A, Abraham K, Weikert C, Kleuser B, Schurmann A, Laeger T (2019) Methionine restriction prevents onset of type 2 diabetes in NZO mice. FASEB J 33(6):7092–7102. https://doi.org/10.1096/fj.201900150R
Olsen T, Ovrebo B, Haj-Yasein N, Lee S, Svendsen K, Hjorth M, Bastani NE, Norheim F, Drevon CA, Refsum H, Vinknes KJ (2020) Effects of dietary methionine and cysteine restriction on plasma biomarkers, serum fibroblast growth factor 21, and adipose tissue gene expression in women with overweight or obesity: a double-blind randomized controlled pilot study. J Transl Med 18(1):122. https://doi.org/10.1186/s12967-020-02288-x
So WY, Cheng Q, Xu A, Lam KS, Leung PS (2015) Loss of fibroblast growth factor 21 action induces insulin resistance, pancreatic islet hyperplasia and dysfunction in mice. Cell Death Dis 6:e1707. https://doi.org/10.1038/cddis.2015.80
Liu JJ, Foo JP, Liu S, Lim SC (2015) The role of fibroblast growth factor 21 in diabetes and its complications: a review from clinical perspective. Diabetes Res Clin Pract 108(3):382–389. https://doi.org/10.1016/j.diabres.2015.02.032
So WY, Leung PS (2016) Fibroblast growth factor 21 as an emerging therapeutic target for type 2 diabetes mellitus. Med Res Rev 36(4):672–704. https://doi.org/10.1002/med.21390
Li YC, Li YZ, Li R, Lan L, Li CL, Huang M, Shi D, Feng RN, Sun CH (2018) Dietary sulfur-containing amino acids are associated with higher prevalence of overweight/obesity in Northern Chinese Adults, an internet-based cross-sectional study. Ann Nutr Metab 73(1):44–53. https://doi.org/10.1159/000490194
Blachier F, Andriamihaja M, Blais A (2020) Sulfur-containing amino acids and lipid metabolism. J Nutr 150(Suppl 1):2524S-2531S. https://doi.org/10.1093/jn/nxaa243
Mirmiran P, Bahadoran Z, Esfandyari S, Azizi F (2017) Dietary protein and amino acid profiles in relation to risk of dysglycemia: findings from a prospective population-based study. Nutrients. https://doi.org/10.3390/nu9090971
Virtanen JK, Voutilainen S, Rissanen TH, Happonen P, Mursu J, Laukkanen JA, Poulsen H, Lakka TA, Salonen JT (2006) High dietary methionine intake increases the risk of acute coronary events in middle-aged men. Nutr Metab Cardiovasc Dis 16(2):113–120
Larsson SC, Hakansson N, Wolk A (2015) Dietary cysteine and other amino acids and stroke incidence in women. Stroke 46(4):922–926. https://doi.org/10.1161/STROKEAHA.114.008022
Mirmiran P, Bahadoran Z, Ghasemi A, Azizi F (2017) Contribution of dietary amino acids composition to incidence of cardiovascular outcomes: a prospective population-based study. Nutr Metab Cardiovasc Dis 27(7):633–641. https://doi.org/10.1016/j.numecd.2017.05.003
Mariotti F (2017) Vegetarian and plant-based diets in health and disease prevention. Academic Press, New York
Acknowledgements
The authors’ responsibilities were as follows: ZD, XG and JPR designed the research, ZD conducted the research and drafted the paper, ZD and VMC analyzed the data and drafted the paper, ZD, XG, JM, RS, RW, and JPR contributed to analysis and manuscript development; and all authors read and approved the final manuscript.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
The NHANES study has been approved by its institutional review board (17) and all procedures have been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Consent ot participate
All persons gave their informed consent prior to their inclusion in the study as approved by the NHANES institutional review board (17).
Consent for publication
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dong, Z., Gao, X., Chinchilli, V.M. et al. Association of dietary sulfur amino acid intake with mortality from diabetes and other causes. Eur J Nutr 61, 289–298 (2022). https://doi.org/10.1007/s00394-021-02641-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-021-02641-w