Abstract
Purpose
To determine the effect of feeding buttermilk-derived choline metabolites on the immune system development in Sprague–Dawley rat pups.
Methods
Sprague–Dawley dams were randomized to one of the three diets containing 1.7 g/kg choline: 1-Control (100% free choline (FC)), 2-Buttermilk (BM, 37% phosphatidylcholine (PC), 34% sphingomyelin (SM), 17% glycerophosphocholine (GPC), 7% FC, 5% phosphocholine), and 3-Placebo (PB, 50% PC, 25% FC, 25% GPC) until the end of the lactation period. At weaning, pups continued on the same diet as their mom. Cell phenotypes and cytokine production by mitogen-stimulated splenocytes isolated from 3- and 10-week-old pups were measured.
Results
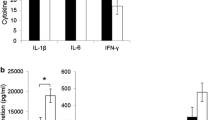
At 3 weeks, BM-pups had a higher proportion of cytotoxic T cells (CTL; CD3 + CD8 +) while both BM- and PB-pups had an increased proportion of cells expressing CD28 + , CD86 + and CD27 + (all p > 0.05). Following ConA stimulation, splenocytes from BM- and PB-pups produced more TNF-α and IFN-γ and after LPS stimulation produced more IL-10 and TNF-α (all p > 0.05). Starting at week 6 of age, BM-pups had a higher body weight. At 10 weeks, both the BM- and PB-pups had a higher proportion of CTL expressing CD27 + . After ConA stimulation, splenocytes from BM- and PB-pups produced more IL-2, IFN-γ and IL-6 and more IL-10 after LPS stimulation (all p > 0.05).
Conclusion
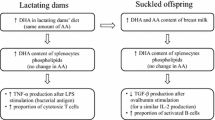
The proportion of lipid soluble forms of choline in the diet during lactation and weaning periods influence the immune system development in rat offspring.

Similar content being viewed by others
Change history
17 October 2023
A Correction to this paper has been published: https://doi.org/10.1007/s00394-023-03262-1
Abbreviations
- AA:
-
Arachidonic acid
- ALA:
-
α-Linolenic acid
- APC:
-
Antigen presenting cells
- BM:
-
Buttermilk diet
- ConA:
-
Concanavalin A
- CTL:
-
Cytotoxic T cells
- CTLA-4:
-
Cytotoxic T lymphocyte associated protein 4
- DHA:
-
Docosahexaenoic acid
- DPA:
-
Docosapentaenoic acid
- FC:
-
Free choline
- GALT:
-
Gut-associated lymphoid tissue
- GPC:
-
Glycerophosphocholine
- IFN-γ:
-
Interferon-gamma
- Ig:
-
Immunoglobulin
- IL:
-
Interleukin
- LPS:
-
Lipopolysaccharide
- MFGM:
-
Milk fat globule membrane
- MUFA:
-
Monounsaturated fatty acid
- n:
-
Omega
- OVA:
-
Ovalbumin
- PB:
-
Placebo diet
- PC:
-
Phosphatidylcholine
- Pcho:
-
Phosphocholine
- PRRs:
-
Pattern recognition receptors
- PUFA:
-
Polyunsaturated fatty acid
- SFA:
-
Saturated fatty acid
- SM:
-
Sphingomyelin
- TCR:
-
T cell receptor
- TLR4:
-
Toll-like receptor 4
- TNF-α:
-
Tumor necrosis factor-alpha
References
Basha S, Surendran N, Pichichero M (2014) Immune responses in neonates. Expert Rev Clin Immunol 10(9):1171–1184. https://doi.org/10.1586/1744666X.2014.942288
Blumer N, Pfefferle PI, Renz H (2007) Development of mucosal immune function in the intrauterine and early postnatal environment. Curr Opin Gastroenterol 23(6):655–660. https://doi.org/10.1097/MOG.0b013e3282eeb428
Peters RL, Dang TD, Allen KJ (2016) Specific oral tolerance induction in childhood. Pediatr Allergy Immunol 27(8):784–794. https://doi.org/10.1111/pai.12620
Pabst O, Mowat AM (2012) Oral tolerance to food protein. Mucosal Immunol 5(3):232–239. https://doi.org/10.1038/mi.2012.4
Yaqoob P, Calder P (2011) The immune and inflammatory SYSTEMS. In: Lanham-New S, MacDonald IM, Roche HM (eds) Nutrition and metabolism. Wiley-Blackwell, Oxford
Prescott SL (2003) Early origins of allergic disease: a review of processes and influences during early immune development. Curr Opin Allergy Clin Immunol 3(2):125–132. https://doi.org/10.1097/01.all.0000064776.57552.32
Hollenbeck CB (2012) An introduction to the nutrition and metabolism of choline. Cent Nerv Syst Agents Med Chem 12(2):100–113. https://doi.org/10.2174/187152412800792689
Zeisel SH (2006) Choline: critical role during fetal development and dietary requirements in adults. Annu Rev Nutr 26:229–250. https://doi.org/10.1146/annurev.nutr.26.061505.111156
Dellschaft NS, Ruth MR, Goruk S, Lewis ED, Richard C, Jacobs RL et al (2015) Choline is required in the diet of lactating dams to maintain maternal immune function. Br J Nutr 113(11):1723–1731. https://doi.org/10.1017/S0007114515001221
Bremer J, Greenberg DM (1961) Methyl transfering enzyme system of microsomes in the biosynthesis of lecithin (phosphatidylcholine). Biochem Biophys Acta 46(2):205–216. https://doi.org/10.1016/0006-3002(61)90745-4
Zhu X, Mar MH, Song J, Zeisel SH (2004) Deletion of the Pemt gene increases progenitor cell mitosis, DNA and protein methylation and decreases calretinin expression in embryonic day 17 mouse hippocampus. Brain Res Dev Brain Res 149(2):121–129. https://doi.org/10.1016/j.devbrainres.2004.01.004
Richard C, Lewis ED, Goruk S, Wadge E, Curtis JM, Jacobs RL et al (2017) Feeding a mixture of choline forms to lactating dams improves the development of the immune system in Sprague-Dawley rat offspring. Nutrients. https://doi.org/10.3390/nu9060567
Lewis ED, Subhan FB, Bell RC, McCargar LJ, Curtis JM, Jacobs RL et al (2014) Estimation of choline intake from 24 h dietary intake recalls and contribution of egg and milk consumption to intake among pregnant and lactating women in Alberta. Br J Nutr 112(1):112–121. https://doi.org/10.1017/S0007114514000555
Vanderghem C, Bodson S, Danthine S, Paquot M, Deroanne C, Blecker C (2010) Milk fat globule membrane and buttermilks: From composition to valorization. Biotechnologie, Agronomie, Société et Environnement, p 14
Conway V, Gauthier SF, Pouliot Y (2014) Buttermilk: much more than a source of milk phospholipids. Animal Front 4(2):44–51. https://doi.org/10.2527/af.2014-0014
Lopez C, Cauty C, Guyomarc’h F (2018) Unraveling the complexity of milk fat globules to tailor bioinspired emulsions providing health benefits: the key role played by the biological membrane. Eur J Lipid Sci Technol. https://doi.org/10.1002/ejlt.201800201
Norris G, Porter C, Jiang C, Blesso CN (2017) Dietary milk sphingomyelin reduces systemic inflammation in diet-induced obese mice and inhibits LPS activity in macrophages. Beverages 3:37. https://doi.org/10.3390/beverages3030037
Park EJ, Suh M, Thomson B, Ma DW, Ramanujam K, Thomson AB et al (2007) Dietary ganglioside inhibits acute inflammatory signals in intestinal mucosa and blood induced by systemic inflammation of Escherichia coli lipopolysaccharide. Shock 28(1):112–117. https://doi.org/10.1097/SHK.0b013e3180310fec
Azarcoya-Barrera J, Goruk S, Lewis ED, Pouliot Y, Curtis JM, Steele R et al (2020) Feeding buttermilk-derived choline forms during gestation and lactation modulates ex vivo T-cell response in rat dams. J Nutr. https://doi.org/10.1093/jn/nxaa089
Field CJ, Wu G, Metroz-Dayer MD, Montambault M, Marliss EB (1990) Lactate production is the major metabolic fate of glucose in splenocytes and is altered in spontaneously diabetic BB rats. Biochem J 272(2):445–452. https://doi.org/10.1097/00005176-200009000-00017
Field CJ, Thomson CA, Van Aerde JE, Parrott A, Euler A, Lien E et al (2000) Lower proportion of CD45R0+ cells and deficient interleukin-10 production by formula-fed infants, compared with human-fed, is corrected with supplementation of long-chain polyunsaturated fatty acids. J Pediatr Gastroenterol Nutr 31(3):291–299
Blewett HJ, Gerdung CA, Ruth MR, Proctor SD, Field CJ (2009) Vaccenic acid favourably alters immune function in obese JCR:LA-cp rats. Br J Nutr 102(4):526–536. https://doi.org/10.1017/S0007114509231722
Reeves PG, Nielsen FH, Fahey GC Jr (1993) AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 123(11):1939–1951. https://doi.org/10.1093/jn/123.11.1939
Kawamura T, Okubo T, Sato K, Fujita S, Goto K, Hamaoka T et al (2012) Glycerophosphocholine enhances growth hormone secretion and fat oxidation in young adults. Nutrition 28(11–12):1122–1126. https://doi.org/10.1016/j.nut.2012.02.011
Vickers MH, Guan J, Gustavsson M, Krageloh CU, Breier BH, Davison M et al (2009) Supplementation with a mixture of complex lipids derived from milk to growing rats results in improvements in parameters related to growth and cognition. Nutr Res 29(6):426–435. https://doi.org/10.1016/j.nutres.2009.06.001
Simon AK, Hollander GA, McMichael A (2015) Evolution of the immune system in humans from infancy to old age. Proc Biol Sci 282(1821):20143085. https://doi.org/10.1098/rspb.2014.3085
Adkins B, Bu Y, Guevara P (2001) The generation of Th memory in neonates versus adults: prolonged primary Th2 effector function and impaired development of Th1 memory effector function in murine neonates. J Immunol 166(2):918–925. https://doi.org/10.4049/jimmunol.166.2.918
Wilson CB, Westall J, Johnston L, Lewis DB, Dower SK, Alpert AR (1986) Decreased production of interferon-gamma by human neonatal cells. Intrinsic and regulatory deficiencies. J Clin Invest 77(3):860–867. https://doi.org/10.1172/JCI112383
Bradley JR (2008) TNF-mediated inflammatory disease. J Pathol 214(2):149–160. https://doi.org/10.1002/path.2287
Billiau A (1996) Interferon-gamma: biology and role in pathogenesis. Adv Immunol 62:61–130. https://doi.org/10.1016/s0065-2776(08)60428-9
Schroder K, Hertzog PJ, Ravasi T, Hume DA (2004) Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol 75(2):163–189. https://doi.org/10.1189/jlb.0603252
Baud V, Karin M (2001) Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol 11(9):372–377. https://doi.org/10.1016/s0962-8924(01)02064-5
Sweet MJ, Hume DA (1996) Endotoxin signal transduction in macrophages. J Leukoc Biol 60(1):8–26. https://doi.org/10.1002/jlb.60.1.8
Goldsby R, Kindt T, Osborne B, Kuby J (2003) Kuby Immunology. W.H. Freeman and Company, USA
Cicchese JM, Evans S, Hult C, Joslyn LR, Wessler T, Millar JA et al (2018) Dynamic balance of pro- and anti-inflammatory signals controls disease and limits pathology. Immunol Rev 285(1):147–167. https://doi.org/10.1111/imr.12671
Couper KN, Blount DG, Riley EM (2008) IL-10: the master regulator of immunity to infection. J Immunol 180(9):5771–5777. https://doi.org/10.4049/jimmunol.180.9.5771
Kasahara T, Hooks JJ, Dougherty SF, Oppenheim JJ (1983) Interleukin 2-mediated immune interferon (IFN-gamma) production by human T cells and T cell subsets. J Immunol 130(4):1784–1789
Dutton RW, Bradley LM, Swain SL (1998) T cell memory. Annu Rev Immunol 16:201–223. https://doi.org/10.1146/annurev.immunol.16.1.201
Reddy M, Eirikis E, Davis C, Davis HM, Prabhakar U (2004) Comparative analysis of lymphocyte activation marker expression and cytokine secretion profile in stimulated human peripheral blood mononuclear cell cultures: an in vitro model to monitor cellular immune function. J Immunol Methods 293(1–2):127–142. https://doi.org/10.1016/j.jim.2004.07.006
Gabay C (2006) Interleukin-6 and chronic inflammation. Arthritis Res Ther 8(Suppl 2):S3. https://doi.org/10.1186/ar1917
Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, O’Garra A (1991) IL-10 inhibits cytokine production by activated macrophages. J Immunol 147(11):3815–3822
Xing Z, Gauldie J, Cox G, Baumann H, Jordana M, Lei XF et al (1998) IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J Clin Invest 101(2):311–320. https://doi.org/10.1172/JCI1368
Walker W, Aste-Amezaga M, Kastelein RA, Trinchieri G, Hunter CA (1999) IL-18 and CD28 use distinct molecular mechanisms to enhance NK cell production of IL-12-induced IFN-gamma. J Immunol 162(10):5894–5901
Ahmad S, Azid NA, Boer JC, Lim J, Chen X, Plebanski M et al (2018) The key role of TNF-TNFR2 interactions in the modulation of allergic inflammation: a review. Front Immunol 9:2572. https://doi.org/10.3389/fimmu.2018.02572
Wawrzyniak M, O’Mahony L, Akdis M (2017) Role of Regulatory Cells in Oral Tolerance. Allergy Asthma Immunol Res 9(2):107–115. https://doi.org/10.4168/aair.2017.9.2.107
Wang X, Sherman A, Liao G, Leong KW, Daniell H, Terhorst C et al (2013) Mechanism of oral tolerance induction to therapeutic proteins. Adv Drug Deliv Rev 65(6):759–773. https://doi.org/10.1016/j.addr.2012.10.013
Wambre E, Jeong D (2018) Oral tolerance development and maintenance. Immunol Allergy Clin North Am 38(1):27–37. https://doi.org/10.1016/j.iac.2017.09.003
Cebra JJ, Jiang HQ, Boiko N, H. T-H. (2005) The role of mucosal microbiota in the development, maintenance, and pathologies of the mucosal immune system. Mucosal Immunol. https://doi.org/10.1016/B978-012491543-5/50022-X
Acknowledgements
The authors would like to acknowledge the technical assistance of Emily Wadge, Reid Steele, Nicole Coursen and Marnie Newell. We express our gratitude to the undergraduate students that worked on the project.
Funding
This study was supported by a Dairy Farmers of Canada grant and Discovery grants from the Natural Sciences and Engineering Research Council of Canada. JAB is recipient of a PhD CONACYT (Consejo Nacional de Ciencia y Tecnología) scholarship from Mexico.
Author information
Authors and Affiliations
Contributions
CR, CJF, RLJ, YP and JMC designed and obtained funding for this study. SG, AM and JAB conducted research and analyzed data. JAB performed the statistical analysis and wrote the manuscript under the supervision of CR and RLJ. CR and CJF have primary responsibility for final content. All authors have read and approved the final manuscript.
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Azarcoya-Barrera, J., Field, C.J., Goruk, S. et al. Buttermilk: an important source of lipid soluble forms of choline that influences the immune system development in Sprague–Dawley rat offspring. Eur J Nutr 60, 2807–2818 (2021). https://doi.org/10.1007/s00394-020-02462-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-020-02462-3