Abstract
Purpose
Aging is known to play a critical role in the etiopathogenesis of several diseases. Among them, cardiovascular disorders are especially relevant since they are becoming the first cause of death in western countries. Resveratrol is a polyphenolic compound that has been shown to exert beneficial effects at different levels, including neuronal and cardiovascular protection. Those effects of resveratrol are related, at least in part, to its antioxidant and anti-inflammatory properties. In the current investigation we were interested in exploring whether the positive effects of resveratrol at cardiac level were taking place even when the supplementation started in already old animals.
Methods
Old male rats were supplemented with resveratrol during 10 weeks. Using RT-PCR, we analyzed the effects of resveratrol supplementation on the expression of different genes related to inflammation, oxidative stress and apoptosis in rat heart.
Results
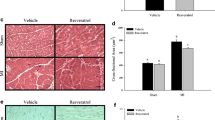
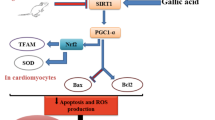
Resveratrol reverted age-related changes in inflammatory, oxidative and apoptotic markers in the rat heart. Among others, the expression of two major inflammatory markers, INF-γ and TNF-α and two oxidative markers, heme oxygenase-1 and nitric oxide synthase, were increased with aging, and resveratrol supplementation reduced the level of some of these to those observed in the heart of young animals. Moreover, age-related changes in apoptotic markers in rat heart tend to be also reverted by resveratrol treatment.
Conclusion
Our results suggest that resveratrol might exert beneficial effects as an anti-aging compound to revert age-related changes in cardiac function.





Similar content being viewed by others
References
Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Mackey JS, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O’Flaherty M, Palaniappan LP, Pandey A, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UKA, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P, American Heart Association Council on E, Prevention Statistics C, Stroke Statistics S (2018) Heart disease and stroke statistics-2018 update: a report from the American heart association. Circulation 137(12):e67–e492. https://doi.org/10.1161/CIR.0000000000000558
Paneni F, Diaz Canestro C, Libby P, Luscher TF, Camici GG (2017) The aging cardiovascular system: understanding it at the cellular and clinical levels. J Am Coll Cardiol 69(15):1952–1967. https://doi.org/10.1016/j.jacc.2017.01.064
Martin-Fernandez B, Gredilla R (2016) Mitochondria and oxidative stress in heart aging. Age (Dordr) 38(4):225–238. https://doi.org/10.1007/s11357-016-9933-y
Matz RL, Schott C, Stoclet JC, Andriantsitohaina R (2000) Age-related endothelial dysfunction with respect to nitric oxide, endothelium-derived hyperpolarizing factor and cyclooxygenase products. Physiol Res 49(1):11–18
Riancho JA, Zarrabeitia MT, Amado JA, Olmos JM, Gonzalez-Macias J (1994) Age-related differences in cytokine secretion. Gerontology 40(1):8–12
Liguori I, Russo G, Curcio F, Bulli G, Aran L, Della-Morte D, Gargiulo G, Testa G, Cacciatore F, Bonaduce D, Abete P (2018) Oxidative stress, aging, and diseases. Clin Interv Aging 13:757–772. https://doi.org/10.2147/CIA.S158513
Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, Hofer T, Seo AY, Sullivan R, Jobling WA, Morrow JD, Van Remmen H, Sedivy JM, Yamasoba T, Tanokura M, Weindruch R, Leeuwenburgh C, Prolla TA (2005) Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science 309(5733):481–484. https://doi.org/10.1126/science.1112125 (309/5733/481 [pii])
Hiona A, Sanz A, Kujoth GC, Pamplona R, Seo AY, Hofer T, Someya S, Miyakawa T, Nakayama C, Samhan-Arias AK, Servais S, Barger JL, Portero-Otin M, Tanokura M, Prolla TA, Leeuwenburgh C (2010) Mitochondrial DNA mutations induce mitochondrial dysfunction, apoptosis and sarcopenia in skeletal muscle of mitochondrial DNA mutator mice. PLoS ONE 5(7):e11468. https://doi.org/10.1371/journal.pone.0011468
Macchi B, Paola DR, Marino-Merlo F, Felice MR, Cuzzocrea S, Mastino A (2015) Inflammatory and cell death pathways in brain and peripheral blood in Parkinson’s disease. CNS Neurol Disord Drug Targets 14(3):313–324
Plesnila N, Zhu C, Culmsee C, Groger M, Moskowitz MA, Blomgren K (2004) Nuclear translocation of apoptosis-inducing factor after focal cerebral ischemia. J Cereb Blood Flow Metab 24(4):458–466. https://doi.org/10.1097/00004647-200404000-00011
Gredilla R, Barja G (2005) Minireview: the role of oxidative stress in relation to caloric restriction and longevity. Endocrinology 146(9):3713–3717. https://doi.org/10.1210/en.2005-0378
Ingram DK, de Cabo R (2017) Calorie restriction in rodents: caveats to consider. Ageing Res Rev 39:15–28. https://doi.org/10.1016/j.arr.2017.05.008
Li J, Zhang CX, Liu YM, Chen KL, Chen G (2017) A comparative study of anti-aging properties and mechanism: resveratrol and caloric restriction. Oncotarget 8(39):65717–65729. https://doi.org/10.18632/oncotarget.20084
Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA (2003) Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 425(6954):191–196. https://doi.org/10.1038/nature01960
Bitterman JL, Chung JH (2015) Metabolic effects of resveratrol: addressing the controversies. Cell Mol Life Sci 72(8):1473–1488. https://doi.org/10.1007/s00018-014-1808-8
Almeida L, Vaz-da-Silva M, Falcao A, Soares E, Costa R, Loureiro AI, Fernandes-Lopes C, Rocha JF, Nunes T, Wright L, Soares-da-Silva P (2009) Pharmacokinetic and safety profile of trans-resveratrol in a rising multiple-dose study in healthy volunteers. Mol Nutr Food Res 53(Suppl 1):S7-15. https://doi.org/10.1002/mnfr.200800177
Cottart CH, Nivet-Antoine V, Laguillier-Morizot C, Beaudeux JL (2010) Resveratrol bioavailability and toxicity in humans. Mol Nutr Food Res 54(1):7–16. https://doi.org/10.1002/mnfr.200900437
Brown VA, Patel KR, Viskaduraki M, Crowell JA, Perloff M, Booth TD, Vasilinin G, Sen A, Schinas AM, Piccirilli G, Brown K, Steward WP, Gescher AJ, Brenner DE (2010) Repeat dose study of the cancer chemopreventive agent resveratrol in healthy volunteers: safety, pharmacokinetics, and effect on the insulin-like growth factor axis. Cancer Res 70(22):9003–9011. https://doi.org/10.1158/0008-5472.CAN-10-2364
Nair AB, Jacob S (2016) A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm 7(2):27–31. https://doi.org/10.4103/0976-0105.177703
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162(1):156–159. https://doi.org/10.1006/abio.1987.9999
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-∆∆C(T)) method. Methods 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Tome-Carneiro J, Gonzalvez M, Larrosa M, Yanez-Gascon MJ, Garcia-Almagro FJ, Ruiz-Ros JA, Tomas-Barberan FA, Garcia-Conesa MT, Espin JC (2013) Resveratrol in primary and secondary prevention of cardiovascular disease: a dietary and clinical perspective. Ann N Y Acad Sci 1290:37–51. https://doi.org/10.1111/nyas.12150
Dolinsky VW (1812) Dyck JR (2011) Calorie restriction and resveratrol in cardiovascular health and disease. Biochim Biophys Acta 11:1477–1489. https://doi.org/10.1016/j.bbadis.2011.06.010
Ramis MR, Esteban S, Miralles A, Tan DX, Reiter RJ (2015) Caloric restriction, resveratrol and melatonin: role of SIRT1 and implications for aging and related-diseases. Mech Ageing Dev 146–148:28–41. https://doi.org/10.1016/j.mad.2015.03.008
Borra MT, Smith BC, Denu JM (2005) Mechanism of human SIRT1 activation by resveratrol. J Biol Chem 280(17):17187–17195. https://doi.org/10.1074/jbc.M501250200
Bagul PK, Dinda AK, Banerjee SK (2015) Effect of resveratrol on sirtuins expression and cardiac complications in diabetes. Biochem Biophys Res Commun 468(1–2):221–227. https://doi.org/10.1016/j.bbrc.2015.10.126
Wan D, Zhou Y, Wang K, Hou Y, Hou R, Ye X (2016) Resveratrol provides neuroprotection by inhibiting phosphodiesterases and regulating the cAMP/AMPK/SIRT1 pathway after stroke in rats. Brain Res Bull 121:255–262. https://doi.org/10.1016/j.brainresbull.2016.02.011
Poulose N (1852) Raju R (2015) Sirtuin regulation in aging and injury. Biochim Biophys Acta 11:2442–2455. https://doi.org/10.1016/j.bbadis.2015.08.017
Price NL, Gomes AP, Ling AJ, Duarte FV, Martin-Montalvo A, North BJ, Agarwal B, Ye L, Ramadori G, Teodoro JS, Hubbard BP, Varela AT, Davis JG, Varamini B, Hafner A, Moaddel R, Rolo AP, Coppari R, Palmeira CM, de Cabo R, Baur JA, Sinclair DA (2012) SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab 15(5):675–690. https://doi.org/10.1016/j.cmet.2012.04.003
Kim I, He YY (2013) Targeting the AMP-activated protein kinase for cancer prevention and therapy. Front Oncol 3:175. https://doi.org/10.3389/fonc.2013.00175
Shackelford DB, Shaw RJ (2009) The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer 9(8):563–575. https://doi.org/10.1038/nrc2676
Zhang J, Zhao P, Quan N, Wang L, Chen X, Cates C, Rousselle T, Li J (2017) The endotoxemia cardiac dysfunction is attenuated by AMPK/mTOR signaling pathway regulating autophagy. Biochem Biophys Res Commun 492(3):520–527. https://doi.org/10.1016/j.bbrc.2017.08.034
Gong H, Pang J, Han Y, Dai Y, Dai D, Cai J, Zhang TM (2014) Age-dependent tissue expression patterns of Sirt1 in senescence-accelerated mice. Mol Med Rep 10(6):3296–3302. https://doi.org/10.3892/mmr.2014.2648
Gines C, Cuesta S, Kireev R, Garcia C, Rancan L, Paredes SD, Vara E, Tresguerres JAF (2017) Protective effect of resveratrol against inflammation, oxidative stress and apoptosis in pancreas of aged SAMP8 mice. Exp Gerontol 90:61–70. https://doi.org/10.1016/j.exger.2017.01.021
Aukrust P, Damas JK, Gullestad L (2003) Immunomodulating therapy: new treatment modality in congestive heart failure. Congest Heart Fail 9(2):64–69
Mann DL (2002) Inflammatory mediators and the failing heart: past, present, and the foreseeable future. Circ Res 91(11):988–998
Kubota T, McTiernan CF, Frye CS, Slawson SE, Lemster BH, Koretsky AP, Demetris AJ, Feldman AM (1997) Dilated cardiomyopathy in transgenic mice with cardiac-specific overexpression of tumor necrosis factor-alpha. Circ Res 81(4):627–635
Pires BRB, Silva R, Ferreira GM, Abdelhay E (2018) NF-kappaB: two sides of the same coin. Genes (Basel) 9(1):1–24. https://doi.org/10.3390/genes9010024
Van der Heiden K, Cuhlmann S, le Luong A, Zakkar M, Evans PC (2010) Role of nuclear factor kappaB in cardiovascular health and disease. Clin Sci (Lond) 118(10):593–605. https://doi.org/10.1042/CS20090557
Ren Z, Wang L, Cui J, Huoc Z, Xue J, Cui H, Mao Q, Yang R (2013) Resveratrol inhibits NF-κB signaling through suppression of p65 and IkappaB kinase activities. Pharmazie 68(8):689–694
Ungvari Z, Sonntag WE, de Cabo R, Baur JA, Csiszar A (2011) Mitochondrial protection by resveratrol. Exerc Sport Sci Rev 39(3):128–132. https://doi.org/10.1097/JES.0b013e3182141f80
Bonnefont-Rousselot D (2016) Resveratrol and cardiovascular diseases. Nutrients 8(5):250. https://doi.org/10.3390/nu8050250
Labinskyy N, Csiszar A, Veress G, Stef G, Pacher P, Oroszi G, Wu J, Ungvari Z (2006) Vascular dysfunction in aging: potential effects of resveratrol, an anti-inflammatory phytoestrogen. Curr Med Chem 13(9):989–996
He JZ, Ho JJ, Gingerich S, Courtman DW, Marsden PA, Ward ME (2010) Enhanced translation of heme oxygenase-2 preserves human endothelial cell viability during hypoxia. J Biol Chem 285(13):9452–9461. https://doi.org/10.1074/jbc.M109.077230
Han F, Takeda K, Yokoyama S, Ueda H, Shinozawa Y, Furuyama K, Shibahara S (2005) Dynamic changes in expression of heme oxygenases in mouse heart and liver during hypoxia. Biochem Biophys Res Commun 338(1):653–659. https://doi.org/10.1016/j.bbrc.2005.08.100
Akasaka E, Takekoshi S, Horikoshi Y, Toriumi K, Ikoma N, Mabuchi T, Tamiya S, Matsuyama T, Ozawa A (2010) Protein oxidative damage and heme oxygenase in sunlight-exposed human skin: roles of MAPK responses to oxidative stress. Tokai J Exp Clin Med 35(4):152–164
Munoz-Sanchez J, Chanez-Cardenas ME (2014) A review on hemeoxygenase-2: focus on cellular protection and oxygen response. Oxid Med Cell Longev 2014:604981. https://doi.org/10.1155/2014/604981
Araujo JA, Zhang M, Yin F (2012) Heme oxygenase-1, oxidation, inflammation, and atherosclerosis. Front Pharmacol 3:119. https://doi.org/10.3389/fphar.2012.00119
Posa A, Szabo R, Csonka A, Veszelka M, Berko AM, Barath Z, Menesi R, Pavo I, Gyongyosi M, Laszlo F, Kupai K, Varga C (2015) Endogenous estrogen-mediated heme oxygenase regulation in experimental menopause. Oxid Med Cell Longev 2015:429713. https://doi.org/10.1155/2015/429713
Marcantoni E, Di Francesco L, Dovizio M, Bruno A, Patrignani P (2012) Novel insights into the vasoprotective role of heme oxygenase-1. Int J Hypertens 2012:127910. https://doi.org/10.1155/2012/127910
Posa A, Kupai K, Menesi R, Szalai Z, Szabo R, Pinter Z, Palfi G, Gyongyosi M, Berko A, Pavo I, Varga C (2013) Sexual dimorphism of cardiovascular ischemia susceptibility is mediated by heme oxygenase. Oxid Med Cell Longev 2013:521563. https://doi.org/10.1155/2013/521563
Forman K, Vara E, Garcia C, Kireev R, Cuesta S, Acuna-Castroviejo D, Tresguerres JA (2010) Beneficial effects of melatonin on cardiological alterations in a murine model of accelerated aging. J Pineal Res 49(3):312–320. https://doi.org/10.1111/j.1600-079X.2010.00800.x
Chen CY, Jang JH, Li MH, Surh YJ (2005) Resveratrol upregulates heme oxygenase-1 expression via activation of NF-E2-related factor 2 in PC12 cells. Biochem Biophys Res Commun 331(4):993–1000. https://doi.org/10.1016/j.bbrc.2005.03.237
Wang L, Li H (2017) effect of resveratrol on the Nrf2 and HO-1 expression in diabetic vascular endothelial cells. Int J Clin Exp Med 10(1):8
Liu PL, Tsai JR, Charles AL, Hwang JJ, Chou SH, Ping YH, Lin FY, Chen YL, Hung CY, Chen WC, Chen YH, Chong IW (2010) Resveratrol inhibits human lung adenocarcinoma cell metastasis by suppressing heme oxygenase 1-mediated nuclear factor-kappaB pathway and subsequently downregulating expression of matrix metalloproteinases. Mol Nutr Food Res 54(Suppl 2):S196-204. https://doi.org/10.1002/mnfr.200900550
Seals DR, Jablonski KL, Donato AJ (2011) Aging and vascular endothelial function in humans. Clin Sci (Lond) 120(9):357–375. https://doi.org/10.1042/CS20100476
Tresguerres JA, Kireev R, Forman K, Cuesta S, Tresguerres AF, Vara E (2012) Effect of chronic melatonin administration on several physiological parameters from old Wistar rats and SAMP8 mice. Curr Aging Sci 5(3):242–253
Cuesta S, Kireev R, Garcia C, Forman K, Escames G, Vara E, Tresguerres JA (2011) Beneficial effect of melatonin treatment on inflammation, apoptosis and oxidative stress on pancreas of a senescence accelerated mice model. Mech Ageing Dev 132(11–12):573–582. https://doi.org/10.1016/j.mad.2011.10.005
Farrokhfall K, Hashtroudi MS, Ghasemi A, Mehrani H (2015) Comparison of inducible nitric oxide synthase activity in pancreatic islets of young and aged rats. Iran J Basic Med Sci 18(2):115–121
Smith CJ, Santhanam L, Bruning RS, Stanhewicz A, Berkowitz DE, Holowatz LA (2011) Upregulation of inducible nitric oxide synthase contributes to attenuated cutaneous vasodilation in essential hypertensive humans. Hypertension 58(5):935–942. https://doi.org/10.1161/HYPERTENSIONAHA.111.178129
de Belder A, Radomski M, Hancock V, Brown A, Moncada S, Martin J (1995) Megakaryocytes from patients with coronary atherosclerosis express the inducible nitric oxide synthase. Arterioscler Thromb Vasc Biol 15(5):637–641
Wilcox JN, Subramanian RR, Sundell CL, Tracey WR, Pollock JS, Harrison DG, Marsden PA (1997) Expression of multiple isoforms of nitric oxide synthase in normal and atherosclerotic vessels. Arterioscler Thromb Vasc Biol 17(11):2479–2488
Yang YM, Huang A, Kaley G, Sun D (2009) eNOS uncoupling and endothelial dysfunction in aged vessels. Am J Physiol Heart Circ Physiol 297(5):H1829-1836. https://doi.org/10.1152/ajpheart.00230.2009
Kumar D, Jugdutt BI (2003) Apoptosis and oxidants in the heart. J Lab Clin Med 142(5):288–297. https://doi.org/10.1016/S0022-2143(03)00148-3
Batkai S, Rajesh M, Mukhopadhyay P, Hasko G, Liaudet L, Cravatt BF, Csiszar A, Ungvari Z, Pacher P (2007) Decreased age-related cardiac dysfunction, myocardial nitrative stress, inflammatory gene expression, and apoptosis in mice lacking fatty acid amide hydrolase. Am J Physiol Heart Circ Physiol 293(2):H909-918. https://doi.org/10.1152/ajpheart.00373.2007
Tower J (2015) Programmed cell death in aging. Ageing Res Rev 23(Pt A):90–100. https://doi.org/10.1016/j.arr.2015.04.002
Phaneuf S, Leeuwenburgh C (2002) Cytochrome c release from mitochondria in the aging heart: a possible mechanism for apoptosis with age. Am J Physiol Regul Integr Comp Physiol 282(2):R423-430. https://doi.org/10.1152/ajpregu.00296.2001
Selman C, Kendaiah S, Gredilla R, Leeuwenburgh C (2003) Increased hepatic apoptosis during short-term caloric restriction is not associated with an enhancement in caspase levels. Exp Gerontol 38(8):897–903
Selman C, Gredilla R, Phaneuf S, Kendaiah S, Barja G, Leeuwenburgh C (2003) Short-term caloric restriction and regulatory proteins of apoptosis in heart, skeletal muscle and kidney of Fischer 344 rats. Biogerontology 4(3):141–147
Kwak HB (2013) Effects of aging and exercise training on apoptosis in the heart. J Exerc Rehabil 9(2):212–219. https://doi.org/10.12965/jer.130002
Holcik M, Lefebvre C, Yeh C, Chow T, Korneluk RG (1999) A new internal-ribosome-entry-site motif potentiates XIAP- mediated cytoprotection. Nat Cell Biol 1(3):190–192. https://doi.org/10.1038/11109
Zhang C, Feng Y, Qu S, Wei X, Zhu H, Luo Q, Liu M, Chen G, Xiao X (2011) Resveratrol attenuates doxorubicin-induced cardiomyocyte apoptosis in mice through SIRT1-mediated deacetylation of p53. Cardiovasc Res 90(3):538–545. https://doi.org/10.1093/cvr/cvr022
Sin TK, Yu AP, Yung BY, Yip SP, Chan LW, Wong CS, Ying M, Rudd JA, Siu PM (2014) Modulating effect of SIRT1 activation induced by resveratrol on Foxo1-associated apoptotic signalling in senescent heart. J Physiol 592(12):2535–2548. https://doi.org/10.1113/jphysiol.2014.271387
Locatelli FM, Kawano T, Iwata H, Aoyama B, Eguchi S, Nishigaki A, Yamanaka D, Tateiwa H, Shigematsu-Locatelli M, Yokoyama M (2018) Resveratrol-loaded nanoemulsion prevents cognitive decline after abdominal surgery in aged rats. J Pharmacol Sci 137(4):395–402. https://doi.org/10.1016/j.jphs.2018.08.006
Tasatargil A, Tanriover G, Barutcigil A, Turkmen E (2019) Protective effect of resveratrol on methylglyoxal-induced endothelial dysfunction in aged rats. Aging Clin Exp Res 31(3):331–338. https://doi.org/10.1007/s40520-018-0986-x
Acknowledgements
This research was supported by a grant from RETICEF (RETICEFRD12/0043/0032) to JAFT.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Torregrosa-Muñumer, R., Vara, E., Fernández-Tresguerres, J. . et al. Resveratrol supplementation at old age reverts changes associated with aging in inflammatory, oxidative and apoptotic markers in rat heart. Eur J Nutr 60, 2683–2693 (2021). https://doi.org/10.1007/s00394-020-02457-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-020-02457-0