Abstract
Purpose
Viscous dietary fiber, functional seeds and ginseng roots have individually been proposed for the management of diabetes. We explored whether their co-administration would improve glycemic control in type 2 diabetes beyond conventional therapy.
Methods
In a randomized, double-blind, controlled trial conducted at two academic centers (Toronto, Canada and Zagreb, Croatia), individuals with type 2 diabetes were assigned to either an active intervention (10 g viscous fiber, 60 g white chia seeds, 1.5 g American and 0.75 g Korean red ginseng extracts), or energy and fiber-matched control (53 g oat bran, 25 g inulin, 25 g maltodextrose and 2.25 g wheat bran) intervention for 24 weeks, while on conventional standard of care. The prespecified primary endpoint was end difference at week 24 in HbA1c, following an intent-to-treat analysis adjusted for center and baseline.
Results
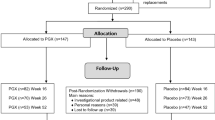
Between January 2016 and April 2018, 104 participants (60M:44F; mean ± SEM age 59 ± 0.8 years; BMI 29.0 ± 0.4 kg/m2; HbA1c 7.0 ± 0.6%) managed with antihyperglycemic agent(s) (n = 98) or lifestyle (n = 6), were randomized (n = 52 test; n = 52 control). At week 24, HbA1c levels were 0.27 ± 0.1% lower on test compared to control (p = 0.03). There was a tendency towards an interaction by baseline HbA1c (p = 0.07), in which a greater reduction was seen in participants with baseline HbA1c > 7% vs ≤ 7% (− 0.56 ± 0.2% vs 0.03 ± 0.2%). Diet and body weight remained unchanged. The interventions were well tolerated with no related adverse events and with high retention rate of 84%.
Conclusions
Co-administration of selected dietary and herbal therapies was well-tolerated and may provide greater glycemic control as add-on therapy in type 2 diabetes.
Registration: Clinicaltrials.gov NCT02553382 (registered on September 17, 2015).



Similar content being viewed by others
Data availability
Data described in the manuscript, code book and analytical code will be made available upon request pending application and approval.
ReferencesPLEASE CORRECT: #25 should read Food and Drug Administration rather than "FaD Administration". And #21 should read "Jovanvoski E, Smircic Duvnjak L, Komishion A,..."
Leiter LA, Berard L, Bowering CK, Cheng AY, Dawson KG, Ekoe JM, Fournier C, Goldin L, Harris SB, Lin P, Ransom T, Tan M, Teoh H, Tsuyuki RT, Whitham D, Woo V, Yale JF, Langer A (2013) Type 2 diabetes mellitus management in Canada: is it improving? Can J Diabetes 37(2):82–89. https://doi.org/10.1016/j.jcjd.2013.02.055
Ryan EA, Pick ME, Marceau C (2001) Use of alternative medicines in diabetes mellitus. Diabet Med 18(3):242–245
Komishon A (2014) The effect of ginseng (Genus Panax) on blood pressure and additional risk factors of cardiovascular disease. University of Toronto, Toronto
Bhardwaj J (2010) The therapeutic effects of the combined use of American ginseng (Panax quinquefolius L.) Extract and Korean Red ginseng (Panax Ginseng C.A. Meyer) extract in the management of type 2 diabetes mellitus and cardiovascular risk factors. University of Toronto, Toronto
Brissette C (2013) The effect of Salvia hispanica L. seeds on weight loss in overweight and obese individuals with type 2 diabetes mellitus. University of Toronto, Toronto
Grossman LD, Roscoe R, Shack AR (2018) Complementary and alternative medicine for diabetes. Can J Diabetes 42(Suppl 1):S154–S161. https://doi.org/10.1016/j.jcjd.2017.10.023
Vuksan V, Jenkins DJ, Spadafora P, Sievenpiper JL, Owen R, Vidgen E, Brighenti F, Josse R, Leiter LA, Bruce-Thompson C (1999) Konjac-mannan (glucomannan) improves glycemia and other associated risk factors for coronary heart disease in type 2 diabetes. A randomized controlled metabolic trial. Diabetes Care 22(6):913–919
Vuksan V, Sievenpiper JL, Owen R, Swilley JA, Spadafora P, Jenkins DJ, Vidgen E, Brighenti F, Josse RG, Leiter LA, Xu Z, Novokmet R (2000) Beneficial effects of viscous dietary fiber from Konjac-mannan in subjects with the insulin resistance syndrome: results of a controlled metabolic trial. Diabetes Care 23(1):9–14
Ho H, Lee AS, Jovanovski E, Jenkins AL, Desouza R, Vuksan V (2013) Effect of whole and ground Salba seeds (Salvia hispanica L.) on postprandial glycemia in healthy volunteers: a randomized controlled, dose-response trial. Eur J Clin Nutr 67(7):786–788. https://doi.org/10.1038/ejcn.2013.103
Vuksan V, Choleva L, Jovanovski E, Jenkins AL, Au-Yeung F, Dias AG, Ho HV, Zurbau A, Duvnjak L (2017) Comparison of flax (Linum usitatissimum) and Salba-chia (Salvia hispanica L.) seeds on postprandial glycemia and satiety in healthy individuals: a randomized, controlled, crossover study. Eur J Clin Nutr 71(2):234–238. https://doi.org/10.1038/ejcn.2016.148
Vuksan V, Choleva L, Perrin-Houdon S, Rogovik A, Fairgrieve C, Jenkins A (2010) improved postprandial glycemia and appetite scores after addition of the ancient grain Salba (Salvia Hispanica L.) vs Flax to an OGTT: possible effect of viscosity. FASEB J. https://doi.org/10.1096/fasebj.24.1_supplement.231.2
Vuksan V, Jenkins AL, Brissette C, Choleva L, Jovanovski E, Gibbs AL, Bazinet RP, Au-Yeung F, Zurbau A, Ho HV, Duvnjak L, Sievenpiper JL, Josse RG, Hanna A (2017) Salba-chia (Salvia hispanica L.) in the treatment of overweight and obese patients with type 2 diabetes: a double-blind randomized controlled trial. Nutr Metab Cardiovasc Dis 27(2):138–146. https://doi.org/10.1016/j.numecd.2016.11.124
Vuksan V, Whitham D, Sievenpiper JL, Jenkins AL, Rogovik AL, Bazinet RP, Vidgen E, Hanna A (2007) Supplementation of conventional therapy with the novel grain Salba (Salvia hispanica L.) improves major and emerging cardiovascular risk factors in type 2 diabetes: results of a randomized controlled trial. Diabetes Care 30(11):2804–2810. https://doi.org/10.2337/dc07-1144
De Souza LR, Jenkins AL, Jovanovski E, Rahelic D, Vuksan V (2015) Ethanol extraction preparation of American ginseng (Panax quinquefolius L) and Korean red ginseng (Panax ginseng C.A. Meyer): differential effects on postprandial insulinemia in healthy individuals. J Ethnopharmacol 159:55–61. https://doi.org/10.1016/j.jep.2014.10.057
Sievenpiper JL, Sung MK, Di Buono M, Seung-Lee K, Nam KY, Arnason JT, Leiter LA, Vuksan V (2006) Korean red ginseng rootlets decrease acute postprandial glycemia: results from sequential preparation- and dose-finding studies. J Am Coll Nutr 25(2):100–107
Vuksan V, Sievenpiper JL, Koo VY, Francis T, Beljan-Zdravkovic U, Xu Z, Vidgen E (2000) American ginseng (Panax quinquefolius L) reduces postprandial glycemia in nondiabetic subjects and subjects with type 2 diabetes mellitus. Arch Intern Med 160(7):1009–1013
Vuksan V, Sievenpiper JL, Wong J, Xu Z, Beljan-Zdravkovic U, Arnason JT, Assinewe V, Stavro MP, Jenkins AL, Leiter LA, Francis T (2001) American ginseng (Panax quinquefolius L.) attenuates postprandial glycemia in a time-dependent but not dose-dependent manner in healthy individuals. Am J Clin Nutr 73(4):753–758
Vuksan V, Stavro MP, Sievenpiper JL, Koo VY, Wong E, Beljan-Zdravkovic U, Francis T, Jenkins AL, Leiter LA, Josse RG, Xu Z (2000) American ginseng improves glycemia in individuals with normal glucose tolerance: effect of dose and time escalation. J Am Coll Nutr 19(6):738–744
Vuksan V, Sung MK, Sievenpiper JL, Stavro PM, Jenkins AL, Di Buono M, Lee KS, Leiter LA, Nam KY, Arnason JT, Choi M, Naeem A (2008) Korean red ginseng (Panax ginseng) improves glucose and insulin regulation in well-controlled, type 2 diabetes: results of a randomized, double-blind, placebo-controlled study of efficacy and safety. Nutr Metab Cardiovasc Dis 18(1):46–56. https://doi.org/10.1016/j.numecd.2006.04.003
Vuksan V, Sievenpiper JL (2005) Herbal remedies in the management of diabetes: lessons learned from the study of ginseng. Nutr Metab Cardiovasc Dis 15(3):149–160. https://doi.org/10.1016/j.numecd.2005.05.001
JJovanvoski E, Smircic Duvnjak L, Komishion A, Blasov K, Au-Yeung F, Sievenpiper JL, Zurbau A, Jenkins AL, Sung MK, Josse RG, Li D, Vuksan V (2019) Effect of co-administration of enriched Korean red ginseng (Panax Ginseng C.A. Meyer) and American Ginseng (Panax quinquefolius L) on cardiometabolic outcomes in type 2 diabetes: a randomized, controlled trial. J Ginseng Res (in press)
Jenkins AL, Morgan LM, Bishop J, Jovanovski E, Jenkins DJA, Vuksan V (2018) Co-administration of a konjac-based fibre blend and American ginseng (Panax quinquefolius L.) on glycaemic control and serum lipids in type 2 diabetes: a randomized controlled, cross-over clinical trial. Eur J Nutr 57(6):2217–2225. https://doi.org/10.1007/s00394-017-1496-x
Jovanovski E, Khayyat R, Zurbau A, Komishon A, Mazhar N, Sievenpiper JL, Blanco Mejia S, Ho HVT, Li D, Jenkins AL, Duvnjak L, Vuksan V (2019) Should viscous fiber supplements be considered in diabetes control? Results from a systematic review and meta-analysis of randomized controlled trials. Diabetes Care 42(5):755–766. https://doi.org/10.2337/dc18-1126
Shishtar E, Sievenpiper JL, Djedovic V, Cozma AI, Ha V, Jayalath VH, Jenkins DJ, Meija SB, de Souza RJ, Jovanovski E, Vuksan V (2014) The effect of ginseng (the genus panax) on glycemic control: a systematic review and meta-analysis of randomized controlled clinical trials. PLoS ONE 9(9):e107391. https://doi.org/10.1371/journal.pone.0107391
Food and Drug Administration (2008) Guidance for industry: Diabetes mellitus. developing drugs and therapeutic biologics for treatment and prevention. FDA, Rockville
Khaw KT, Wareham N, Luben R, Bingham S, Oakes S, Welch A, Day N (2001) Glycated haemoglobin, diabetes, and mortality in men in Norfolk cohort of European prospective investigation of cancer and nutrition (EPIC-Norfolk). BMJ 322(7277):15–18. https://doi.org/10.1136/bmj.322.7277.15
Bloomgarden ZT, Dodis R, Viscoli CM, Holmboe ES, Inzucchi SE (2006) Lower baseline glycemia reduces apparent oral agent glucose-lowering efficacy: a meta-regression analysis. Diabetes Care 29(9):2137–2139. https://doi.org/10.2337/dc06-1120
Jenkins DJ, Wolever TM, Leeds AR, Gassull MA, Haisman P, Dilawari J, Goff DV, Metz GL, Alberti KG (1978) Dietary fibres, fibre analogues, and glucose tolerance: importance of viscosity. Br Med J 1(6124):1392–1394
Teoh SL, Lai NM, Vanichkulpitak P, Vuksan V, Ho H, Chaiyakunapruk N (2018) Clinical evidence on dietary supplementation with chia seed (Salvia hispanica L.): a systematic review and meta-analysis. Nutr Rev 76(4):219–242. https://doi.org/10.1093/nutrit/nux071
Vuksan V, Jenkins AL, Dias AG, Lee AS, Jovanovski E, Rogovik AL, Hanna A (2010) Reduction in postprandial glucose excursion and prolongation of satiety: possible explanation of the long-term effects of whole grain Salba (Salvia Hispanica L.). Eur J Clin Nutr 64(4):436–438. https://doi.org/10.1038/ejcn.2009.159
Vuksan V, Xu ZZ, Jovanovski E, Jenkins AL, Beljan-Zdravkovic U, Sievenpiper JL, Mark Stavro P, Zurbau A, Duvnjak L, Li MZC (2018) Efficacy and safety of American ginseng (Panax quinquefolius L.) extract on glycemic control and cardiovascular risk factors in individuals with type 2 diabetes: a double-blind, randomized, cross-over clinical trial. Eur J Nutr. https://doi.org/10.1007/s00394-018-1642-0
Luo JZ, Luo L (2006) American ginseng stimulates insulin production and prevents apoptosis through regulation of uncoupling protein-2 in cultured beta cells. Evid Based Complement Alternat Med 3(3):365–372. https://doi.org/10.1093/ecam/nel026
Chan CB, MacDonald PE, Saleh MC, Johns DC, Marban E, Wheeler MB (1999) Overexpression of uncoupling protein 2 inhibits glucose-stimulated insulin secretion from rat islets. Diabetes 48(7):1482–1486
Zhang CY, Baffy G, Perret P, Krauss S, Peroni O, Grujic D, Hagen T, Vidal-Puig AJ, Boss O, Kim YB, Zheng XX, Wheeler MB, Shulman GI, Chan CB, Lowell BB (2001) Uncoupling protein-2 negatively regulates insulin secretion and is a major link between obesity, beta cell dysfunction, and type 2 diabetes. Cell 105(6):745–755
Hagen T, Vidal-Puig A (2002) Mitochondrial uncoupling proteins in human physiology and disease. Minerva Med 93(1):41–57
Rousset S, Alves-Guerra MC, Mozo J, Miroux B, Cassard-Doulcier AM, Bouillaud F, Ricquier D (2004) The biology of mitochondrial uncoupling proteins. Diabetes 53(Suppl 1):S130-135
Langin D (2003) The role of uncoupling protein 2 in the development of type 2 diabetes. Drugs Today (Barc) 39(4):287–295
Bang H, Kwak JH, Ahn HY, Shin DY, Lee JH (2014) Korean red ginseng improves glucose control in subjects with impaired fasting glucose, impaired glucose tolerance, or newly diagnosed type 2 diabetes mellitus. J Med Food 17(1):128–134. https://doi.org/10.1089/jmf.2013.2889
Kim HOPM, Han JS (2011) Effects of fermented red ginseng supplementation on blood glucose and insulin resistance in type 2 diabetic patients. J Korean Soc Food Sci Nutr 40(5):696–703
Mohanan P, Subramaniyam S, Mathiyalagan R, Yang DC (2018) Molecular signaling of ginsenosides Rb1, Rg1, and Rg3 and their mode of actions. J Ginseng Res 42(2):123–132. https://doi.org/10.1016/j.jgr.2017.01.008
Pan C, Guo Q, Lu N (2019) Role of gut microbiota in the pharmacological effects of natural products. Evid Based Complement Alternat Med 2019:2682748. https://doi.org/10.1155/2019/2682748
Song MY, Kim BS, Kim H (2014) Influence of Panax ginseng on obesity and gut microbiota in obese middle-aged Korean women. J Ginseng Res 38(2):106–115. https://doi.org/10.1016/j.jgr.2013.12.004
Guo M, Ding S, Zhao C, Gu X, He X, Huang K, Luo Y, Liang Z, Tian H, Xu W (2015) Red Ginseng and Semen Coicis can improve the structure of gut microbiota and relieve the symptoms of ulcerative colitis. J Ethnopharmacol 162:7–13. https://doi.org/10.1016/j.jep.2014.12.029
Zhou P, Xie W, He S, Sun Y, Meng X, Sun G, Sun X (2019) Ginsenoside Rb1 as an anti-diabetic agent and its underlying mechanism analysis. Cells. https://doi.org/10.3390/cells8030204
Kim DH (2018) Gut microbiota-mediated pharmacokinetics of ginseng saponins. J Ginseng Res 42(3):255–263. https://doi.org/10.1016/j.jgr.2017.04.011
Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) UK Prospective Diabetes Study (UKPDS) Group (1998). Lancet 352 (9131):837–853
Dascalu A, Sievenpiper JL, Jenkins AL, Stavro MP, Leiter LA, Arnason JT, Vuksan V (2007) Five batches representative of Ontario-grown American ginseng root produce comparable reductions of postprandial glycemia in healthy individuals. Can J Physiol Pharmacol 85(9):856–864. https://doi.org/10.1139/y07-030
Funding
Funding for this study was provided from Diabetes Canada, Banting and Best Diabetes Centre, and BTGin Co., Ltd., Korea. JLS was funded by a Diabetes Canada Clinician Scientist Award.
Author information
Authors and Affiliations
Contributions
AZ conducted the research (Toronto), data analysis and wrote the manuscript. LSD supervised the research (Croatia) and reviewed/edited the manuscript. SM and JM conducted the research (Croatia). EJ conducted the research (Toronto) and reviewed/edited the manuscript. ALJ, DJAJ RJ, LAL and JLS reviewed/edited the manuscript. VV conceptualized and designed the study, supervised the research at both study sites and reviewed/edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
AZ is a part-time employee at INQUIS Clinical Research, Ltd., a contract research organization. LSD served as advisor or speaker for Astrazeneca, Boehringer, Lilly, NovoNordisk, Novartis, MSD, Merck, Sanofi, Takeda. ALJ is a part owner and Director of Research at INQUIS Clinical Research, Ltd., a contract research organization. DJAJ has received research grants from Saskatchewan & Alberta Pulse Growers Associations, the Agricultural Bioproducts Innovation Program through the Pulse Research Network, the Advanced Foods and Material Network, Loblaw Companies Ltd., Unilever Canada and Netherlands, Barilla, the Almond Board of California, Agriculture and Agri-food Canada, Pulse Canada, Kellogg's Company, Canada, Quaker Oats, Canada, Procter & Gamble Technical Centre Ltd., Bayer Consumer Care, Springfield, NJ, Pepsi/Quaker, International Nut & Dried Fruit (INC), Soy Foods Association of North America, the Coca-Cola Company (investigator initiated, unrestricted grant), Solae, Haine Celestial, the Sanitarium Company, Orafti, the International Tree Nut Council Nutrition Research and Education Foundation, the Peanut Institute, Soy Nutrition Institute (SNI), the Canola and Flax Councils of Canada, the Calorie Control Council, the Canadian Institutes of Health Research (CIHR), the Canada Foundation for Innovation (CFI) and the Ontario Research Fund (ORF). He has received in-kind supplies for trials as a research support from the Almond board of California, Walnut Council of California, American Peanut Council, Barilla, Unilever, Unico, Primo, Loblaw Companies, Quaker (Pepsico), Pristine Gourmet, Bunge Limited, Kellogg Canada, WhiteWave Foods. He has been on the speaker's panel, served on the scientific advisory board and/or received travel support and/or honoraria from the Almond Board of California, Canadian Agriculture Policy Institute, Loblaw Companies Ltd, the Griffin Hospital (for the development of the NuVal scoring system), the Coca-Cola Company, EPICURE, Danone, Diet Quality Photo Navigation (DQPN), Better Therapeutics (FareWell), Verywell, True Health Initiative (THI), Heali AI Corp, Institute of Food Technologists (IFT), Soy Nutrition Institute (SNI), Herbalife Nutrition Institute (HNI), Saskatchewan & Alberta Pulse Growers Associations, Sanitarium Company, Orafti, the American Peanut Council, the International Tree Nut Council Nutrition Research and Education Foundation, the Peanut Institute, Herbalife International, Pacific Health Laboratories, Nutritional Fundamentals for Health (NFH), Barilla, Metagenics, Bayer Consumer Care, Unilever Canada and Netherlands, Solae, Kellogg, Quaker Oats, Procter & Gamble, Abbott Laboratories, Dean Foods, the California Strawberry Commission, Haine Celestial, PepsiCo, the Alpro Foundation, Pioneer Hi-Bred International, DuPont Nutrition and Health, Spherix Consulting and WhiteWave Foods, the Advanced Foods and Material Network, the Canola and Flax Councils of Canada, Agri-Culture and Agri-Food Canada, the Canadian Agri-Food Policy Institute, Pulse Canada, the Soy Foods Association of North America, the Nutrition Foundation of Italy (NFI), Nutra-Source Diagnostics, the McDougall Program, the Toronto Knowledge Translation Group (St. Michael's Hospital), the Canadian College of Naturopathic Medicine, The Hospital for Sick Children, the Canadian Nutrition Society (CNS), the American Society of Nutrition (ASN), Arizona State University, Paolo Sorbini Foundation and the Institute of Nutrition, Metabolism and Diabetes. He received an honorarium from the United States Department of Agriculture to present the 2013 W.O. Atwater Memorial Lecture. He received the 2013 Award for Excellence in Research from the International Nut and Dried Fruit Council. He received funding and travel support from the Canadian Society of Endocrinology and Metabolism to produce mini cases for the Canadian Diabetes Association (CDA). He is a member of the International Carbohydrate Quality Consortium (ICQC). His wife, Alexandra L Jenkins, is a director and partner of INQUIS Clinical Research for the Food Industry, his 2 daughters, Wendy Jenkins and Amy Jenkins, have published a vegetarian book that promotes the use of the foods described here, The Portfolio Diet for Cardiovascular Risk Reduction (Academic Press/Elsevier 2020 ISBN:978-0-12-810510-8) and his sister, Caroline Brydson, received funding through a grant from the St. Michael's Hospital Foundation to develop a cookbook for one of his studies. JLS has received research support from the Canadian Foundation for Innovation, Ontario Research Fund, Province of Ontario Ministry of Research and Innovation and Science, Canadian Institutes of health Research (CIHR), Diabetes Canada, PSI Foundation, Banting and Best Diabetes Centre (BBDC), American Society for Nutrition (ASN), INC International Nut and Dried Fruit Council Foundation, National Dried Fruit Trade Association, The Tate and Lyle Nutritional Research Fund at the University of Toronto, The Glycemic Control and Cardiovascular Disease in Type 2 Diabetes Fund at the University of Toronto (a fund established by the Alberta Pulse Growers), and the Nutrition Trialists Fund at the University of Toronto (a fund established by an inaugural donation from the Calorie Control Council). He has received in-kind food donations to support a randomized controlled trial from the Almond Board of California, California Walnut Commission, American Peanut Council, Barilla, Unilever, Unico/Primo, Loblaw Companies, Quaker, Kellogg Canada, and WhiteWave Foods. He has received travel support, speaker fees and/or honoraria from Diabetes Canada, Mott’s LLP, Dairy Farmers of Canada, FoodMinds LLC, International Sweeteners Association, Nestlé, Pulse Canada, Canadian Society for Endocrinology and Metabolism (CSEM), GI Foundation, Abbott, Biofortis, ASN, Northern Ontario School of Medicine, INC Nutrition Research & Education Foundation, European Food Safety Authority (EFSA), Comité Européen des Fabricants de Sucre (CEFS), and Physicians Committee for Responsible Medicine. He has or has had ad hoc consulting arrangements with Perkins Coie LLP, Tate & Lyle, and Wirtschaftliche Vereinigung Zucker e.V. He is a member of the European Fruit Juice Association Scientific Expert Panel and Soy Nutrition Institute (SNI) Scientific Advisory Committee. He is on the Clinical Practice Guidelines Expert Committees of Diabetes Canada, European Association for the study of Diabetes (EASD), Canadian Cardiovascular Society (CCS), and Obesity Canada. He serves or has served as an unpaid scientific advisor for the Food, Nutrition, and Safety Program (FNSP) and the Technical Committee on Carbohydrates of the International Life Science Institute (ILSI) North America. He is a member of the International Carbohydrate Quality Consortium (ICQC), Executive Board Member of the Diabetes and Nutrition Study Group (DNSG) of the EASD, and Director of the Toronto 3D Knowledge Synthesis and Clinical Trials foundation. His wife is an employee of AB InBev. VV was partial owner of Glycemic Index Laboratories Inc. (Toronto, Canada) during 2004–2015 and previously held the Canadian (2,410,556) and American (7,326,404) patent on the medical use of viscous fiber blend for reducing blood glucose for treatment of diabetes mellitus, increasing insulin sensitivity, and reducing systolic blood pressure and blood lipids. SM, EJ, JM, LAL and RGJ declare no conflict of interest related to this study.
Ethical approval
The study was conducted in accordance with Good Clinical Practice Guidelines and approved by the ethics boards of St. Michael’s Hospital, UH Merkur and School of Medicine, University of Zagreb.
Consent to participate
Individuals who were interested in participating provided written informed consent.
Consent for publication
All authors have approved the final version of the manuscript. We attest that the contents of this manuscript are our original work. These study findings have been presented at the 36th International Symposium on Diabetes and Nutrition, an annual symposium organized by the Diabetes and Nutrition Study Group of the European Association for the Study of Diabetes.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zurbau, A., Smircic Duvnjak, L., Magas, S. et al. Co-administration of viscous fiber, Salba-chia and ginseng on glycemic management in type 2 diabetes: a double-blind randomized controlled trial. Eur J Nutr 60, 3071–3083 (2021). https://doi.org/10.1007/s00394-020-02434-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-020-02434-7