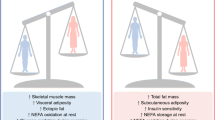
Abstract
Purpose
Obesity and high-fat (HF) diet are associated with over activation of the endocannabinoid system (ECS). We have demonstrated that maternal HF diet induces early obesity and modulates cannabinoid signaling in visceral (VIS) and subcutaneous (SUB) white adipose tissue (WAT) in weanling rat offspring. We hypothesized that perinatal maternal HF diet would program the expression of ECS in adipose tissue in a long-term way in parallel to alterations in epigenetic markers and sex hormone signaling.
Methods
Progenitor female rats received control diet (C, 9% fat) or isocaloric high-fat diet (HF, 28% fat) for 8 weeks before mating, gestation, and lactation. All pups were weaned to C diet and they were euthanized at 180 days old.
Results
Maternal HF diet induced overweight and increased SUB WAT mass of male and female adult offspring. Maternal HF diet induced hypertrophy of VIS and SUB adipocytes only in female offspring associated with increased type 1 cannabinoid receptor protein (CB1) and mRNA (Cnr1) levels. These changes were associated with increased estrogen receptor α binding to Cnr1 promoter in SUB WAT of adult female offspring, which may contribute to higher expression of Cnr1.
Conclusion
Increased CB1 signaling in adipose tissue might contribute to higher adiposity programmed by maternal HF diet because endocannabinoids stimulate the accumulation of fat in the adipose tissue. Our findings provide molecular insights into sex-specific targets for anti-obesity therapies based on the endocannabinoid system.







Similar content being viewed by others
References
Lillycrop KA, Burdge GC (2015) Maternal diet as a modifier of offspring epigenetics. J Dev Orig Health Dis 6(2):88–95. https://doi.org/10.1017/S2040174415000124
Stang J, Huffman LG (2016) Position of the academy of nutrition and dietetics: obesity, reproduction, and pregnancy outcomes. J Acad Nutr Diet 116(4):677–691. https://doi.org/10.1016/j.jand.2016.01.008
Joss-Moore LA, Lane RH, Albertine KH (2015) Epigenetic contributions to the developmental origins of adult lung disease. Biochem Cell Biol 93(2):119–127. https://doi.org/10.1139/bcb-2014-0093
Burgio E, Lopomo A, Migliore L (2015) Obesity and diabetes: from genetics to epigenetics. Mol Biol Rep 42(4):799–818. https://doi.org/10.1007/s11033-014-3751-z
Mancuso P, Bouchard B (2019) The impact of aging on adipose function and adipokine synthesis. Front Endocrinol (Lausanne) 10:137. https://doi.org/10.3389/fendo.2019.00137
Frank AP, de Souza SR, Palmer BF, Clegg DJ (2018) Determinants of body fat distribution in humans may provide insight about obesity-related health risks. J Lipid Res. https://doi.org/10.1194/jlr.R086975
Ibrahim MM (2010) Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev 11(1):11–18. https://doi.org/10.1111/j.1467-789X.2009.00623.x
Cristino L, Becker T, Di Marzo V (2014) Endocannabinoids and energy homeostasis: an update. BioFactors 40(4):389–397. https://doi.org/10.1002/biof.1168
D'Addario C, Micioni Di Bonaventura MV, Pucci M, Romano A, Gaetani S, Ciccocioppo R, Cifani C, Maccarrone M (2014) Endocannabinoid signaling and food addiction. Neurosci Biobehav Rev 47:203–224. https://doi.org/10.1016/j.neubiorev.2014.08.008
Matias I, Di Marzo V (2007) Endocannabinoids and the control of energy balance. Trends Endocrinol Metab 18(1):27–37. https://doi.org/10.1016/j.tem.2006.11.006
Maccarrone M, Bab I, Biro T, Cabral GA, Dey SK, Di Marzo V, Konje JC, Kunos G, Mechoulam R, Pacher P, Sharkey KA, Zimmer A (2015) Endocannabinoid signaling at the periphery: 50 years after THC. Trends Pharmacol Sci 36(5):277–296. https://doi.org/10.1016/j.tips.2015.02.008
Silvestri C, Di Marzo V (2013) The endocannabinoid system in energy homeostasis and the etiopathology of metabolic disorders. Cell Metab 17(4):475–490. https://doi.org/10.1016/j.cmet.2013.03.001
Verty AN, Stefanidis A, McAinch AJ, Hryciw DH, Oldfield B (2015) Anti-obesity effect of the CB2 receptor agonist JWH-015 in diet-induced obese Mice. PLoS ONE 10(11):e0140592. https://doi.org/10.1371/journal.pone.0140592
Chiurchiu V, Lanuti M, Catanzaro G, Fezza F, Rapino C, Maccarrone M (2014) Detailed characterization of the endocannabinoid system in human macrophages and foam cells, and anti-inflammatory role of type-2 cannabinoid receptor. Atherosclerosis 233(1):55–63. https://doi.org/10.1016/j.atherosclerosis.2013.12.042
Ueda Y, Miyagawa N, Wakitani K (2007) Involvement of cannabinoid CB2 receptors in the IgE-mediated triphasic cutaneous reaction in mice. Life Sci 80(5):414–419. https://doi.org/10.1016/j.lfs.2006.09.026
Deveaux V, Cadoudal T, Ichigotani Y, Teixeira-Clerc F, Louvet A, Manin S, Nhieu JT, Belot MP, Zimmer A, Even P, Cani PD, Knauf C, Burcelin R, Bertola A, Le Marchand-Brustel Y, Gual P, Mallat A, Lotersztajn S (2009) Cannabinoid CB2 receptor potentiates obesity-associated inflammation, insulin resistance and hepatic steatosis. PLoS ONE 4(6):e5844. https://doi.org/10.1371/journal.pone.0005844
Christensen R, Kristensen PK, Bartels EM, Bliddal H, Astrup A (2007) Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomised trials. Lancet 370(9600):1706–1713. https://doi.org/10.1016/S0140-6736(07)61721-8
Ramirez-Lopez MT, Arco R, Decara J, Vazquez M, Noemi Blanco R, Alen F, Suarez J, Gomez de Heras R, Rodriguez de Fonseca F (2016) Exposure to a highly caloric palatable diet during the perinatal period affects the expression of the endogenous cannabinoid system in the brain, liver and adipose tissue of adult rat offspring. PLoS ONE 11(11):e0165432. https://doi.org/10.1371/journal.pone.0165432
Ramirez-Lopez MT, Arco R, Decara J, Vazquez M, Rivera P, Blanco RN, Alen F, Gomez de Heras R, Suarez J, Rodriguez de Fonseca F (2016) Long-term effects of prenatal exposure to undernutrition on cannabinoid receptor-related behaviors: sex and tissue-specific alterations in the mRNA expression of cannabinoid receptors and lipid metabolic regulators. Front Behav Neurosci 10:241. https://doi.org/10.3389/fnbeh.2016.00241
Ramirez-Lopez MT, Vazquez M, Bindila L, Lomazzo E, Hofmann C, Blanco RN, Alen F, Anton M, Decara J, Ouro D, Orio L, Suarez J, Lutz B, Rodriguez de Fonseca F, Gomez de Heras R (2015) Exposure to a highly caloric palatable diet during pregestational and gestational periods affects hypothalamic and hippocampal endocannabinoid levels at birth and induces adiposity and anxiety-like behaviors in male rat offspring. Front Behav Neurosci 9:339. https://doi.org/10.3389/fnbeh.2015.00339
Almeida MM, Dias-Rocha CP, Souza AS, Muros MF, Mendonca LS, Pazos-Moura CC, Trevenzoli IH (2017) Perinatal maternal high-fat diet induces early obesity and sex-specific alterations of the endocannabinoid system in white and brown adipose tissue of weanling rat offspring. Br J Nutr 118(10):788–803. https://doi.org/10.1017/S0007114517002884
Dias-Rocha CP, Almeida MM, Santana EM, Costa JCB, Franco JG, Pazos-Moura CC, Trevenzoli IH (2018) Maternal high-fat diet induces sex-specific endocannabinoid system changes in newborn rats and programs adiposity, energy expenditure and food preference in adulthood. J Nutr Biochem 51:56–68. https://doi.org/10.1016/j.jnutbio.2017.09.019
Almeida MM, Dias-Rocha CP, Reis-Gomes CF, Wang H, Atella GC, Cordeiro A, Pazos-Moura CC, Joss-Moore L, Trevenzoli IH (2019) Maternal high-fat diet impairs leptin signaling and up-regulates type-1 cannabinoid receptor with sex-specific epigenetic changes in the hypothalamus of newborn rats. Psychoneuroendocrinology 103:306–315. https://doi.org/10.1016/j.psyneuen.2019.02.004
Miranda RA, De Almeida MM, Rocha C, de Brito FL, De Souza LL, Souza AFP, Andrade CBV, Fortunato RS, Pazos-Moura CC, Trevenzoli IH (2018) Maternal high-fat diet consumption induces sex-dependent alterations of the endocannabinoid system and redox homeostasis in liver of adult rat offspring. Sci Rep 8(1):14751. https://doi.org/10.1038/s41598-018-32906-0
Souza AFP, Souza LL, Oliveira LS, Cordeiro A, Souza E, Kluck GEG, Atella GC, Trevenzoli IH, Pazos-Moura CC (2019) Fish oil supplementation during adolescence attenuates metabolic programming of perinatal maternal high-fat diet in adult offspring. Br J Nutr. https://doi.org/10.1017/S0007114519000771
Franco JG, Dias-Rocha CP, Fernandes TP, Albuquerque Maia L, Lisboa PC, Moura EG, Pazos-Moura CC, Trevenzoli IH (2016) Resveratrol treatment rescues hyperleptinemia and improves hypothalamic leptin signaling programmed by maternal high-fat diet in rats. Eur J Nutr 55(2):601–610. https://doi.org/10.1007/s00394-015-0880-7
Franco JG, Fernandes TP, Rocha CP, Calvino C, Pazos-Moura CC, Lisboa PC, Moura EG, Trevenzoli IH (2012) Maternal high-fat diet induces obesity and adrenal and thyroid dysfunction in male rat offspring at weaning. J Physiol 590(21):5503–5518. https://doi.org/10.1113/jphysiol.2012.240655
Reeves PG, Nielsen FH, Fahey GC Jr (1993) AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 123(11):1939–1951. https://doi.org/10.1093/jn/123.11.1939
Fischbeck KL, Rasmussen KM (1987) Effect of repeated reproductive cycles on maternal nutritional status, lactational performance and litter growth in ad libitum-fed and chronically food-restricted rats. J Nutr 117(11):1967–1975. https://doi.org/10.1093/jn/117.11.1967
Osman OS, Selway JL, Kepczynska MA, Stocker CJ, O'Dowd JF, Cawthorne MA, Arch JR, Jassim S, Langlands K (2013) A novel automated image analysis method for accurate adipocyte quantification. Adipocyte 2(3):160–164. https://doi.org/10.4161/adip.24652
Joss-Moore LA, Wang Y, Ogata EM, Sainz AJ, Yu X, Callaway CW, McKnight RA, Albertine KH, Lane RH (2011) IUGR differentially alters MeCP2 expression and H3K9Me3 of the PPARγ gene in male and female rat lungs during alveolarization. Birth Defects Res A Clin Mol Teratol 91(8):672–681. https://doi.org/10.1002/bdra.20783
Karpe F, Pinnick KE (2015) Biology of upper-body and lower-body adipose tissue–link to whole-body phenotypes. Nat Rev Endocrinol 11(2):90–100. https://doi.org/10.1038/nrendo.2014.185
Oliveira LS, Caetano B, Miranda RA, Souza AFP, Cordeiro A, Woyames J, Andrade CBV, Atella GC, Takiya CM, Fortunato RS, Trevenzoli IH, Souza LL, Pazos-Moura CC (2020) Differentiated hepatic response to fructose intake during adolescence reveals the increased susceptibility to non-alcoholic fatty liver disease of maternal high-fat diet male rat offspring. Mol Nutr Food Res 64(3):e1900838. https://doi.org/10.1002/mnfr.201900838
Di Francesco A, Falconi A, Di Germanio C, Micioni Di Bonaventura MV, Costa A, Caramuta S, Del Carlo M, Compagnone D, Dainese E, Cifani C, Maccarrone M, D'Addario C (2015) Extravirgin olive oil up-regulates CB(1) tumor suppressor gene in human colon cancer cells and in rat colon via epigenetic mechanisms. J Nutr Biochem 26(3):250–258. https://doi.org/10.1016/j.jnutbio.2014.10.013
Murdolo G, Kempf K, Hammarstedt A, Herder C, Smith U, Jansson PA (2007) Insulin differentially modulates the peripheral endocannabinoid system in human subcutaneous abdominal adipose tissue from lean and obese individuals. J Endocrinol Invest 30(8):RC17–RC21. https://doi.org/10.1007/BF03347440
Cable JC, Tan GD, Alexander SP, O'Sullivan SE (2011) The activity of the endocannabinoid metabolising enzyme fatty acid amide hydrolase in subcutaneous adipocytes correlates with BMI in metabolically healthy humans. Lipids Health Dis 10:129. https://doi.org/10.1186/1476-511X-10-129
Simeoni U, Armengaud JB, Siddeek B, Tolsa JF (2018) Perinatal origins of adult disease. Neonatology 113(4):393–399. https://doi.org/10.1159/000487618
Brenseke B, Prater MR, Bahamonde J, Gutierrez JC (2013) Current thoughts on maternal nutrition and fetal programming of the metabolic syndrome. J Pregnancy 2013:368461. https://doi.org/10.1155/2013/368461
Seisenberger S, Peat JR, Hore TA, Santos F, Dean W, Reik W (2013) Reprogramming DNA methylation in the mammalian life cycle: building and breaking epigenetic barriers. Philos Trans R Soc Lond B Biol Sci 368(1609):20110330. https://doi.org/10.1098/rstb.2011.0330
Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, Blomqvist L, Hoffstedt J, Naslund E, Britton T, Concha H, Hassan M, Ryden M, Frisen J, Arner P (2008) Dynamics of fat cell turnover in humans. Nature 453(7196):783–787. https://doi.org/10.1038/nature06902
Wierman ME (2007) Sex steroid effects at target tissues: mechanisms of action. Adv Physiol Educ 31(1):26–33. https://doi.org/10.1152/advan.00086.2006
Palmer BF, Clegg DJ (2015) The sexual dimorphism of obesity. Mol Cell Endocrinol 402:113–119. https://doi.org/10.1016/j.mce.2014.11.029
Blouin K, Boivin A, Tchernof A (2008) Androgens and body fat distribution. J Steroid Biochem Mol Biol 108(3–5):272–280. https://doi.org/10.1016/j.jsbmb.2007.09.001
Davis KE, Neinast MD, Sun K, Skiles WM, Bills JD, Zehr JA, Zeve D, Hahner LD, Cox DW, Gent LM, Xu Y (2013) The sexually dimorphic role of adipose and adipocyte estrogen receptors in modulating adipose tissue expansion, inflammation, and fibrosis. Mol Metab 2(3):227–242. https://doi.org/10.1016/j.molmet.2013.05.006
Proto MC, Gazzerro P, Di Croce L, Santoro A, Malfitano AM, Pisanti S, Laezza C, Bifulco M (2012) Interaction of endocannabinoid system and steroid hormones in the control of colon cancer cell growth. J Cell Physiol 227(1):250–258. https://doi.org/10.1002/jcp.22727
Grimaldi P, Pucci M, Di Siena S, Di Giacomo D, Pirazzi V, Geremia R, Maccarrone M (2012) The faah gene is the first direct target of estrogen in the testis: role of histone demethylase LSD1. Cell Mol Life Sci 69(24):4177–4190. https://doi.org/10.1007/s00018-012-1074-6
Lee KS, Asgar J, Zhang Y, Chung MK, Ro JY (2013) The role of androgen receptor in transcriptional modulation of cannabinoid receptor type 1 gene in rat trigeminal ganglia. Neuroscience 254:395–403. https://doi.org/10.1016/j.neuroscience.2013.09.014
Greenbaum D, Colangelo C, Williams K, Gerstein M (2003) Comparing protein abundance and mRNA expression levels on a genomic scale. Genome Biol 4(9):117. https://doi.org/10.1186/gb-2003-4-9-117
Gowran A, Murphy CE, Campbell VA (2009) Delta(9)-tetrahydrocannabinol regulates the p53 post-translational modifiers Murine double minute 2 and the Small Ubiquitin MOdifier protein in the rat brain. FEBS Lett 583(21):3412–3418. https://doi.org/10.1016/j.febslet.2009.09.056
Maia J, Almada M, Silva A, Correia-da-Silva G, Teixeira N, Sa SI, Fonseca BM (2017) The endocannabinoid system expression in the female reproductive tract is modulated by estrogen. J Steroid Biochem Mol Biol 174:40–47. https://doi.org/10.1016/j.jsbmb.2017.07.023
Davis SR, Castelo-Branco C, Chedraui P, Lumsden MA, Nappi RE, Shah D, Villaseca P, Writing Group of the International Menopause Society for World Menopause D (2012) Understanding weight gain at menopause. Climacteric 15(5):419–429. https://doi.org/10.3109/13697137.2012.707385
Iwasa T, Matsuzaki T, Yano K, Irahara M (2018) The effects of ovariectomy and lifelong high-fat diet consumption on body weight, appetite, and lifespan in female rats. Horm Behav 97:25–30. https://doi.org/10.1016/j.yhbeh.2017.10.005
Acknowledgements
We would like to thank Luela Dias and Juliana Penna for the technical support.
Funding
This work was supported by the Carlos Chagas Filho Research Foundation of the State of Rio de Janeiro (FAPERJ) (Grant No. E-26/202.816/2015), Brazil, National Council for Scientific and Technological Development (CNPq) (Grant No. 797473807/2013-0), Brazil, Coordination for the Improvement of Higher Education Personnel (CAPES), Brazil, and National Institutes Health (Grant No. DK084036), United States.
Author information
Authors and Affiliations
Contributions
MMA, CPD-R and CFR-G have participated in animal experiments, collection and interpretation of data. MMA, HW and LJ-M contributed with the chromatin immunoprecipitation assays. IHT, CCP-M, AC delineated the experimental design and supervised the study. MMA, LJ-M and IHT have written the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
de Almeida, M.M., Dias-Rocha, C.P., Reis-Gomes, C.F. et al. Maternal high-fat diet up-regulates type-1 cannabinoid receptor with estrogen signaling changes in a sex- and depot- specific manner in white adipose tissue of adult rat offspring. Eur J Nutr 60, 1313–1326 (2021). https://doi.org/10.1007/s00394-020-02318-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-020-02318-w