Abstract
Purpose
To reveal the mechanism that links industrial trans fatty acids (iTFAs) to various chronic diseases, we examined the impact of iTFAs on the local microenvironment of the small intestine (duodenum, jejunum and ileum).
Methods
Forty male 8-week-old mice were fed diets containing one of the following: (1) low soybean oil (LS); (2) high soybean oil (HS); (3) low partially hydrogenated oil (LH), and (4) high partially hydrogenated oil (HH). The analysis of microbiota from small intestinal content was performed by real-time qPCR. The fatty acid composition of small intestine mucosa was measured by GC/MS, and comparative transcriptome of the small intestinal mucosa was analyzed by RNA-sequencing.
Results
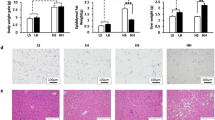
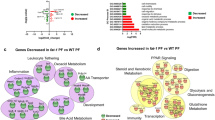
The intake of iTFAs changed the fatty acid spectrum of the small intestine mucosa, especially the excessive accumulation of iTFA (mainly elaidic acid). For microbiota, the relative abundance of δ- and γ-proteobacteria, Lactobacillus, Desulfovibrio, Peptostreptococcus and Turicibacter were significantly different in the iTFA diet groups compared to the control group. Based on the identification of differently expressed genes(DEGs) and pathway annotation, comparative transcriptome analysis of the small intestine mucosa revealed obvious overexpression of genes involved in the extracellular matrix (ECM)-receptor interaction and the peroxisome proliferator-activated receptor signaling pathway, which suggests that ECM remodeling and abnormal lipid metabolism may have occurred with iTFA ingestion.
Conclusion
Our research demonstrated multiple adverse effects of iTFA that may have originated from the small intestine. This finding could be to facilitate the development of new strategies to suppress iTFA-related diseases by reversing the adverse effects of iTFA on intestinal health.






Similar content being viewed by others
References
Dawczynski C, Lorkowski S (2016) Trans-fatty acids and cardiovascular risk: does origin matter? Expert Rev Cardiovasc Ther 14(9):1001–1005. https://doi.org/10.1080/14779072.2016.1199956
Craig-Schmidt MC (2006) World-wide consumption of trans fatty acids. Atheroscler Suppl 7(2):1–4. https://doi.org/10.1016/j.atherosclerosissup.2006.04.001
Engberink MF, Geleijnse JM, Wanders AJ, Brouwer IA (2012) The effect of conjugated linoleic acid, a natural trans fat from milk and meat, on human blood pressure: results from a randomized crossover feeding study. J Hum Hypertens 26(2):127–132. https://doi.org/10.1038/jhh.2010.132
Okada Y, Tsuzuki Y, Ueda T, Hozumi H, Sato S, Hokari R, Kurihara C, Watanabe C, Tomita K, Komoto S, Kawaguchi A, Nagao S, Miura S (2013) Trans fatty acids in diets act as a precipitating factor for gut inflammation? J Gastroenterol Hepatol 28(4):29–32. https://doi.org/10.1111/jgh.12270
Wilczek MM, Olszewski R, Krupienicz A (2017) Trans-fatty acids and cardiovascular disease: urgent need for legislation. Cardiology 138(4):254–258. https://doi.org/10.1159/000479956
van de Vijver LP, Kardinaal AF, Couet C, Aro A, Kafatos A, Steingrimsdottir L, Amorim Cruz JA, Moreiras O, Becker W, van Amelsvoort JM, Vidal-Jessel S, Salminen I, Moschandreas J, Sigfusson N, Martins I, Carbajal A, Ytterfors A, Poppel G (2000) Association between trans fatty acid intake and cardiovascular risk factors in Europe: the TRANSFAIR study. Eur J Clin Nutr 54(2):126–135. https://doi.org/10.1038/sj.ejcn.1600906
Lichtenstein AH (2000) Trans fatty acids and cardiovascular disease risk. Curr Opin Lipidol 11(1):37–42. https://doi.org/10.1097/00041433-200002000-00006
Vinikoor LC, Satia JA, Schroeder JC, Millikan RC, Martin CF, Ibrahim JG, Sandler RS (2009) Associations between trans fatty acid consumption and colon cancer among Whites and African Americans in the North Carolina colon cancer study I. Nutr Cancer 61(4):427–436. https://doi.org/10.1080/01635580802710725
Theodoratou E, McNeill G, Cetnarskyj R, Farrington SM, Tenesa A, Barnetson R, Porteous M, Dunlop M, Campbell H (2007) Dietary fatty acids and colorectal cancer: a case-control study. Am J Epidemiol 166(2):181–195. https://doi.org/10.1093/aje/kwm063
Yu LC (2018) Microbiota dysbiosis and barrier dysfunction in inflammatory bowel disease and colorectal cancers: exploring a common ground hypothesis. J Biomed Sci 25(1):79. https://doi.org/10.1186/s12929-018-0483-8
Barlow GM, Yu A, Mathur R (2015) Role of the gut microbiome in obesity and diabetes mellitus. Nutr Clin Pract 30(6):787–797. https://doi.org/10.1177/0884533615609896
Poirier H, Degrace P, Niot I, Bernard A, Besnard P (1996) Localization and regulation of the putative membrane fatty-acid transporter (FAT) in the small intestine. Comparison with fatty acid-binding proteins (FABP). Eur J Biochem 238(2):368–373. https://doi.org/10.1111/j.1432-1033.1996.0368z.x
Ge Y, Liu W, Tao H, Zhang Y, Liu L, Liu Z, Qiu B, Xu T (2018) Effect of industrial trans-fatty acids-enriched diet on gut microbiota of C57BL/6 mice. Eur J Nutr. https://doi.org/10.1007/s00394-018-1810-2
Kim D, Langmead B, Salzberg SL (2015) HISAT: a fast spliced aligner with low memory requirements. Nat Methods 12(4):357–360. https://doi.org/10.1038/nmeth.3317
Roberts A, Pimentel H, Trapnell C, Pachter L (2011) Identification of novel transcripts in annotated genomes using RNA-Seq. Bioinformatics 27(17):2325–2329. https://doi.org/10.1093/bioinformatics/btr355
Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL, Pachter L (2012) Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc 7(3):562–578. https://doi.org/10.1038/nprot.2012.016
Trapnell C, Hendrickson DG, Sauvageau M, Goff L, Rinn JL, Pachter L (2013) Differential analysis of gene regulation at transcript resolution with RNA-seq. Nat Biotechnol 31(1):46–53. https://doi.org/10.1038/nbt.2450
Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B (2008) Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods 5(7):621–628. https://doi.org/10.1038/nmeth.1226
Chevalier C, Stojanovic O, Colin DJ, Suarez-Zamorano N, Tarallo V, Veyrat-Durebex C, Rigo D, Fabbiano S, Stevanovic A, Hagemann S, Montet X, Seimbille Y, Zamboni N, Hapfelmeier S, Trajkovski M (2015) Gut microbiota orchestrates energy homeostasis during cold. Cell 163(6):1360–1374. https://doi.org/10.1016/j.cell.2015.11.004
Woolf TB, Tychko M (1998) Simulations of fatty acid-binding proteins. II. Sites for discrimination of monounsaturated ligands. Biophys J 74(2 Pt 1):694–707. https://doi.org/10.1016/S0006-3495(98)73995-5
Richieri GV, Ogata RT, Kleinfeld AM (1994) Equilibrium constants for the binding of fatty acids with fatty acid-binding proteins from adipocyte, intestine, heart, and liver measured with the fluorescent probe ADIFAB. J Biol Chem 269(39):23918–23930
Mukhopadhya I, Hansen R, El-Omar EM, Hold GL (2012) IBD-what role do proteobacteria play? Nat Rev Gastroenterol Hepatol 9(4):219–230. https://doi.org/10.1038/nrgastro.2012.14
Shin NR, Whon TW, Bae JW (2015) Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol 33(9):496–503. https://doi.org/10.1016/j.tibtech.2015.06.011
Litvak Y, Byndloss MX, Tsolis RM, Baumler AJ (2017) Dysbiotic proteobacteria expansion: a microbial signature of epithelial dysfunction. Curr Opin Microbiol 39:1–6. https://doi.org/10.1016/j.mib.2017.07.003
Carvalho FA, Koren O, Goodrich JK, Johansson ME, Nalbantoglu I, Aitken JD, Su Y, Chassaing B, Walters WA, Gonzalez A, Clemente JC, Cullender TC, Barnich N, Darfeuille-Michaud A, Vijay-Kumar M, Knight R, Ley RE, Gewirtz AT (2012) Transient inability to manage proteobacteria promotes chronic gut inflammation in TLR5-deficient mice. Cell Host Microbe 12(2):139–152. https://doi.org/10.1016/j.chom.2012.07.004
Endo Y, Kamisada S, Fujimoto K, Saito T (2006) Trans fatty acids promote the growth of some Lactobacillus strains. J Gen Appl Microbiol 52(1):29–35
Ijssennagger N, van der Meer R, van Mil SWC (2016) Sulfide as a mucus barrier-breaker in inflammatory bowel disease? Trends Mol Med 22(3):190–199. https://doi.org/10.1016/j.molmed.2016.01.002
Zhong Y, Nyman M, Fak F (2015) Modulation of gut microbiota in rats fed high-fat diets by processing whole-grain barley to barley malt. Mol Nutr Food Res 59(10):2066–2076. https://doi.org/10.1002/mnfr.201500187
Li TT, Tong AJ, Liu YY, Huang ZR, Wan XZ, Pan YY, Jia RB, Liu B, Chen XH, Zhao C (2019) Polyunsaturated fatty acids from microalgae Spirulina platensis modulates lipid metabolism disorders and gut microbiota in high-fat diet rats. Food Chem Toxicol 131:110558. https://doi.org/10.1016/j.fct.2019.06.005
Remely M, Hippe B, Zanner J, Aumueller E, Brath H, Haslberger AG (2016) Gut microbiota of obese, type 2 diabetic individuals is enriched in Faecalibacterium prausnitzii, Akkermansia muciniphila and Peptostreptococcus anaerobius after weight loss. Endocr Metab Immune Disord Drug Targets 16(2):99–106. https://doi.org/10.2174/1871530316666160831093813
Petrey AC, de la Motte CA (2017) The extracellular matrix in IBD: a dynamic mediator of inflammation. Curr Opin Gastroenterol 33(4):234–238. https://doi.org/10.1097/MOG.0000000000000368
Bonnans C, Chou J, Werb Z (2014) Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol 15(12):786–801. https://doi.org/10.1038/nrm3904
Ingle SB, Adgaonkar BD, Ingle CR (2014) Microscopic colitis: Common cause of unexplained nonbloody diarrhea. World J Gastrointest Pathophysiol 5(1):48–53. https://doi.org/10.4291/wjgp.v5.i1.48
Rieder F, Fiocchi C (2009) Intestinal fibrosis in IBD—a dynamic, multifactorial process. Nat Rev Gastroenterol Hepatol 6(4):228–235. https://doi.org/10.1038/nrgastro.2009.31
Lau E, Marques C, Pestana D, Santoalha M, Carvalho D, Freitas P, Calhau C (2016) The role of I-FABP as a biomarker of intestinal barrier dysfunction driven by gut microbiota changes in obesity. Nutr Metab (Lond) 13:31. https://doi.org/10.1186/s12986-016-0089-7
Stevens BR, Goel R, Seungbum K, Richards EM, Holbert RC, Pepine CJ, Raizada MK (2018) Increased human intestinal barrier permeability plasma biomarkers zonulin and FABP2 correlated with plasma LPS and altered gut microbiome in anxiety or depression. Gut 67(8):1555–1557. https://doi.org/10.1136/gutjnl-2017-314759
Her GM, Yeh YH, Wu JL (2004) Functional conserved elements mediate intestinal-type fatty acid binding protein (I-FABP) expression in the gut epithelia of zebrafish larvae. Dev Dyn 230(4):734–742. https://doi.org/10.1002/dvdy.20081
Stoffel W, Holz B, Jenke B, Binczek E, Gunter RH, Kiss C, Karakesisoglou I, Thevis M, Weber AA, Arnhold S, Addicks K (2008) Delta6-desaturase (FADS2) deficiency unveils the role of omega3- and omega6-polyunsaturated fatty acids. EMBO J 27(17):2281–2292. https://doi.org/10.1038/emboj.2008.156
Melanson EL, Astrup A, Donahoo WT (2009) The relationship between dietary fat and fatty acid intake and body weight, diabetes, and the metabolic syndrome. Ann Nutr Metab 55(1–3):229–243. https://doi.org/10.1159/000229004
Pan A, Chen M, Chowdhury R, Wu JH, Sun Q, Campos H, Mozaffarian D, Hu FB (2012) alpha-Linolenic acid and risk of cardiovascular disease: a systematic review and meta-analysis. Am J Clin Nutr 96(6):1262–1273. https://doi.org/10.3945/ajcn.112.044040
Da Silva MS, Julien P, Bilodeau JF, Barbier O, Rudkowska I (2017) Trans fatty acids suppress TNF-alpha-Induced inflammatory gene expression in endothelial (HUVEC) and hepatocellular carcinoma (HepG2) cells. Lipids 52(4):315–325. https://doi.org/10.1007/s11745-017-4243-4
Dobrzyn P, Sampath H, Dobrzyn A, Miyazaki M, Ntambi JM (2008) Loss of stearoyl-CoA desaturase 1 inhibits fatty acid oxidation and increases glucose utilization in the heart. Am J Physiol Endocrinol Metab 294(2):E357–364. https://doi.org/10.1152/ajpendo.00471.2007
Ikeda J, Ichiki T, Takahara Y, Kojima H, Sankoda C, Kitamoto S, Tokunou T, Sunagawa K (2015) PPARgamma agonists attenuate palmitate-induced ER stress through up-regulation of SCD-1 in macrophages. PLoS ONE 10(6):e0128546. https://doi.org/10.1371/journal.pone.0128546
Marchioro L, Hellmuth C, Uhl O, Geraghty AA, O'Brien EC, Horan MK, Donnelly JM, Kirchberg FF, Koletzko B, McAuliffe FM (2019) Associations of maternal and fetal SCD-1 markers with infant anthropometry and maternal diet: findings from the ROLO study. Clin Nutr. https://doi.org/10.1016/j.clnu.2019.08.030
Liao C, Li M, Li X, Li N, Zhao X, Wang X, Song Y, Quan J, Cheng C, Liu J, Bode AM, Cao Y, Luo X (2019) Trichothecin inhibits invasion and metastasis of colon carcinoma associating with SCD-1-mediated metabolite alteration. Biochim Biophys Acta Mol Cell Biol Lipids 2019:158540. https://doi.org/10.1016/j.bbalip.2019.158540
Liu XL, Cao HX, Wang BC, Xin FZ, Zhang RN, Zhou D, Yang RX, Zhao ZH, Pan Q, Fan JG (2017) miR-192-5p regulates lipid synthesis in non-alcoholic fatty liver disease through SCD-1. World J Gastroenterol 23(46):8140–8151. https://doi.org/10.3748/wjg.v23.i46.8140
Acknowledgements
This research is supported by Key Research and Development Plan of Shandong Province, Grant number: 2018YYSP010; and Special foundation for Taishan Scholars tsqn20161067.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The manuscript does not contain clinical studies or patient data.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, C., Zhang, Y., Ge, Y. et al. Comparative transcriptome and microbiota analyses provide new insights into the adverse effects of industrial trans fatty acids on the small intestine of C57BL/6 mice. Eur J Nutr 60, 975–987 (2021). https://doi.org/10.1007/s00394-020-02297-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-020-02297-y