Abstract
Purpose
The relationships between gut microbiota and obesity-related co-morbidities have been increasingly recognized. Low-grade inflammation may be the main factor in the pathogenesis of such disorders. We investigated the effect of the potential probiotic Bifidobacterium pseudocatenulatum CECT 7765 on cardiometabolic risk factors, inflammatory cytokines and gut microbiota composition in obese children with insulin resistance.
Methods
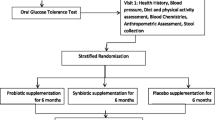
The study included 48 obese children (10–15 years old) with insulin resistance. They received dietary advice and were assigned to take the capsules with or without probiotic (109−10 CFU) daily for 13 weeks. Clinical, biochemical and gut microbiome measurements were made at baseline and at the end of the intervention.
Results
There was a significant improvement in body mass index in all children after the intervention, suggesting that weight changes are related to the dietary advice. A significant decrease in circulating high-sensitive C-reactive protein (P = 0.026) and monocyte chemoattractant protein-1 (P = 0.032) and an increase in high-density lipoprotein cholesterol (P = 0.035) and omentin-1 (P = 0.023) in children receiving probiotic supplementation were observed compared to the control group. Regarding gut microbiota, probiotic administration significantly increased the proportion of the Rikenellaceae family members, particularly of the Alistipes genus.
Conclusions
The beneficial effects of the intervention on inflammatory markers and lipid profile suggest that B. pseudocatenulatum CECT 7765 intake together with dietary recommendations can improve inflammatory status in children with obesity and insulin resistance. These effects are parallel to increases in bacterial groups associated with a lean phenotype. The modulation of gut microbiota with probiotic supplementation can be considered an effective tool to ameliorate some obesity-related disorders in children.



Similar content being viewed by others
References
Roth CL, Jain V (2018) Rising obesity in children: a serious public health concern. Indian J Pediatr 85:461–462. https://doi.org/10.1007/s12098-018-2639-7
Aleksandrova K, Mozaffarian D, Pischon T (2018) Addressing the perfect storm: biomarkers in obesity and pathophysiology of cardiometabolic risk. Clin Chem 64:142–153. https://doi.org/10.1373/clinchem.2017.275172
Reho JJ, Rahmouni K (2017) Oxidative and inflammatory signals in obesity-associated vascular abnormalities. Clin Sci 131:1689–1700. https://doi.org/10.1042/CS20170219
Hernández-Díaz A, Arana-Martínez JC, Carbó R, Espinosa-Cervantes R, Sánchez-Muñoz F (2016) Omentin: role in insulin resistance, inflammation and cardiovascular protection. Arch Cardiol Mex 86:233–243. https://doi.org/10.1016/j.acmx.2015.09.010
Rosenbaum M, Knight R, Leibel RL (2015) The gut microbiota in human energy homeostasis and obesity. Trends Endocrinol Metab 26:493–501. https://doi.org/10.1016/j.tem.2015.07.002
Garcia-Rios A, Torres-Peña JD, Perez-Jimenez F, Perez-Martinez P (2017) Gut microbiota: a new marker of cardiovascular disease. Curr Pharm Des 23:3233–3238. https://doi.org/10.2174/1381612823666170317144853
Yoo J, Kim S (2016) Probiotics and prebiotics: present status and future perspectives on metabolic disorders. Nutrients 8:173. https://doi.org/10.3390/nu8030173
Tonucci LB, Olbrich dos Santos KM, Licursi de Oliveira L, Rocha Ribeiro SM, Duarte Martino HS (2017) Clinical application of probiotics in type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled study. Clin Nutr 36:85–92. https://doi.org/10.1016/j.clnu.2015.11.011
Hendijani F, Akbari V (2018) Probiotic supplementation for management of cardiovascular risk factors in adults with type II diabetes: a systematic review and meta-analysis. Clin Nutr 37:532–541. https://doi.org/10.1016/j.clnu.2017.02.015
Agusti A, Moya-Pérez A, Campillo I, Montserrat-de la Paz S, Cerrudo V, Perez-Villalba A, Sanz Y (2018) Bifidobacterium pseudocatenulatum CECT 7765 ameliorates neuroendocrine alterations associated with an exaggerated stress response and anhedonia in obese mice. Mol Neurobiol 55:5337–5352. https://doi.org/10.1007/s12035-017-0768-z
Cano PG, Santacruz A, Trejo FM, Sanz Y (2013) Bifidobacterium CECT 7765 improves metabolic and immunological alterations associated with obesity in high-fat diet-fed mice. Obesity 21:2310–2321. https://doi.org/10.1002/oby.20330
Mauricio MD, Serna E, Fernández-Murga ML, Portero J, Aldasoro M, Valles SL, Sanz Y, Vila JM (2017) Bifidobacterium pseudocatenulatum CECT 7765 supplementation restores altered vascular function in an experimental model of obese mice. Int J Med Sci 14:444–451. https://doi.org/10.7150/ijms.18354
Moya-Pérez A, Neef A, Sanz Y (2015) Bifidobacterium pseudocatenulatum CECT 7765 reduces obesity-associated inflammation by restoring the lymphocyte-macrophage balance and gut microbiota structure in high-fat diet-fed mice. PLoS One 10:e0126976. https://doi.org/10.1371/journal.pone.0126976
Moya-Pérez A, Romo-Vaquero M, Tomás-Barberán F, Sanz Y, García-Conesa M-T (2014) Hepatic molecular responses to Bifidobacterium pseudocatenulatum CECT 7765 in a mouse model of diet-induced obesity. Nutr Metab Cardiovasc Dis 24:57–64. https://doi.org/10.1016/j.numecd.2013.04.011
Kazama K, Usui T, Okada M, Hara Y, Yamawaki H (2012) Omentin plays an anti-inflammatory role through inhibition of TNF-α-induced superoxide production in vascular smooth muscle cells. Eur J Pharmacol 686:116–123. https://doi.org/10.1016/j.ejphar.2012.04.033
Shibata R, Ouchi N, Ohashi K, Murohara T (2017) The role of adipokines in cardiovascular disease. J Cardiol 70:329–334. https://doi.org/10.1016/j.jjcc.2017.02.006
Deshmane SL, Kremlev S, Amini S, Sawaya BE (2009) Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res 29:313–326. https://doi.org/10.1089/jir.2008.0027
Bruun JM, Lihn AS, Pedersen SB, Richelsen B (2005) Monocyte chemoattractant protein-1 release is higher in visceral than subcutaneous human adipose tissue (AT): implication of macrophages resident in the AT. J Clin Endocrinol Metab 90:2282–2289. https://doi.org/10.1210/jc.2004-1696
García Cuartero B, García Lacalle C, Jiménez Lobo C, González Vergaz A, Calvo Rey C, Alcázar Villar MJ, Díaz Martínez E (2007) The HOMA and QUICKI indexes, and insulin and C-peptide levels in healthy children. Cut off points to identify metabolic syndrome in healthy children. An Pediatr (Barc) 66:481–490. https://doi.org/10.1157/13102513
Keskin M, Kurtoglu S, Kendirci M, Atabek ME, Yazici C (2005) Homeostasis model assessment is more reliable than the fasting glucose/insulin ratio and quantitative insulin sensitivity check index for assessing insulin resistance among obese children and adolescents. Pediatrics 115:e500–e503. https://doi.org/10.1542/peds.2004-1921
de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J (2007) Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ 85:660–667. https://doi.org/10.2471/BLT.07.043497
Mendonça RD, Pimenta AM, Gea A, de la Fuente-Arrillaga C, Martinez-Gonzalez MA, Lopes AC, Bes-Rastrollo M (2016) Ultraprocessed food consumption and risk of overweight and obesity: the University of Navarra Follow-Up (SUN) cohort study. Am J Clin Nutr 104:1433–1440. https://doi.org/10.3945/ajcn.116.135004
Klindworth A, Pruesse E, Schweer T, Peplies J, Quast C, Horn M, Glöckner FO (2013) Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res 41:e1. https://doi.org/10.1093/nar/gks808
Magoc T, Salzberg SL (2011) FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27:2957–2963. https://doi.org/10.1093/bioinformatics/btr507
Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, Van Horn DJ, Weber CF (2009) Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75:7537–7541. https://doi.org/10.1128/AEM.01541-09
Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R (2011) UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27:2194–2200. https://doi.org/10.1093/bioinformatics/btr381
Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glöckner FO (2012) The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 41:D590–D596. https://doi.org/10.1093/nar/gks1219
Wang Q, Garrity GM, Tiedje JM, Cole JR (2007) Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 73:5261–5267. https://doi.org/10.1128/AEM.00062-07
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336. https://doi.org/10.1038/nmeth.f.303
Gøbel RJ, Larsen N, Jakobsen M, Mølgaard C, Michaelsen KF (2012) Probiotics to adolescents with obesity. J Pediatr Gastroenterol Nutr 55:673–678. https://doi.org/10.1097/MPG.0b013e318263066c
Zabetian-Targhi F, Mahmoudi MJ, Rezaei N, Mahmoudi M (2015) Retinol binding protein 4 in relation to diet, inflammation, immunity, and cardiovascular diseases. Adv Nutr 6:748–762. https://doi.org/10.3945/an.115.008292
Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, Kotani K, Quadro L, Kahn BB (2005) Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature 436:356–362. https://doi.org/10.1038/nature03711
Thushara RM, Gangadaran S, Solati Z, Moghadasian MH (2016) Cardiovascular benefits of probiotics: a review of experimental and clinical studies. Food Funct 7:632–642. https://doi.org/10.1039/C5FO01190F
Rader DJ, Hovingh GK (2014) HDL and cardiovascular disease. Lancet 384:618–625. https://doi.org/10.1016/S0140-6736(14)61217-4
Dali-Youcef N, Mecili M, Ricci R, Andrès E (2013) Metabolic inflammation: connecting obesity and insulin resistance. Ann Med 45:242–253. https://doi.org/10.3109/07853890.2012.705015
Mazidi M, Rezaie P, Ferns GA, Vatanparast H (2017) Impact of probiotic administration on serum C-reactive protein concentrations: systematic review and meta-analysis of randomized control trials. Nutrients 9:20. https://doi.org/10.3390/nu9010020
Buyukinan M, Atar M, Can U, Pirgon O, Guzelant A, Deniz I (2018) The association between serum vaspin and omentin-1 levels in obese children with metabolic syndrome. Metab Syndr Relat Disord 16:76–81. https://doi.org/10.1089/met.2017.0133
Moreno-Navarrete JM, Ortega F, Castro A, Sabater M, Ricart W, Fernández-Real JM (2011) Circulating omentin as a novel biomarker of endothelial dysfunction. Obesity 19:1552–1559. https://doi.org/10.1038/oby.2010.351
Cox MA, Jackson J, Stanton M, Rojas-Triana A, Bober L, Laverty M, Yang X, Zhu F, Liu J, Wang S, Monsma F, Vassileva G, Maguire M, Gustafson E, Bayne M, Chou CC, Lundell D, Jenh CH (2009) Short-chain fatty acids act as antiinflammatory mediators by regulating prostaglandin E(2) and cytokines. World J Gastroenterol 15:5549–5557. https://doi.org/10.3748/wjg.15.5549
Moya-Pérez A, Perez-Villalba A, Benítez-Páez A, Campillo I, Sanz Y (2017) Bifidobacterium CECT 7765 modulates early stress-induced immune, neuroendocrine and behavioral alterations in mice. Brain Behav Immun 65:43–56. https://doi.org/10.1016/j.bbi.2017.05.011
Aguirre M, Bussolo de Souza C, Venema K (2016) The gut microbiota from lean and obese subjects contribute differently to the fermentation of arabinogalactan and inulin. PLoS One 11:e0159236. https://doi.org/10.1371/journal.pone.0159236
Acknowledgements
This study was supported by Grant AGL2014-52101-P from the Spanish Ministry of Economy and Competitiveness. The contract of Jesús Sanchis-Jordá was supported by a grant from the Spanish Training University Lecturers Programme (Formación de Profesorado Universitario) from the Spanish Ministry of Education, Culture and Sports (Grant number FPU13/03753). The contracts of Eva M. Gómez del Pulgar and Alfonso Benítez-Páez were supported by the EU Project MyNewGut (no. 613979) from the 7th Framework Program.
Author information
Authors and Affiliations
Contributions
JS-C, PC-F, and JC-L performed the children enrollment, dietary counseling during the study, and statistical analysis of clinical data. EMGP and AB-P accomplished the analysis of gut microbiota. YS and PC-F designed and directed the study.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The protocol of the study was approved by the Dr. Peset University Hospital ethics committee. All procedures in the study were in complete accordance with the Helsinki declaration and its later amendments.
Rights and permissions
About this article
Cite this article
Sanchis-Chordà, J., del Pulgar, E.M.G., Carrasco-Luna, J. et al. Bifidobacterium pseudocatenulatum CECT 7765 supplementation improves inflammatory status in insulin-resistant obese children. Eur J Nutr 58, 2789–2800 (2019). https://doi.org/10.1007/s00394-018-1828-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-018-1828-5