Abstract
Purpose
The association between dietary acrylamide intake and estrogen receptor-positive (ER+) breast cancer risk in epidemiological studies is inconsistent. By analyzing gene-acrylamide interactions for ER+ breast cancer risk, we aimed to clarify the role of acrylamide intake in ER+ breast cancer etiology.
Methods
The prospective Netherlands Cohort Study on diet and cancer includes 62,573 women, aged 55–69 years. At baseline, a random subcohort of 2589 women was sampled from the total cohort for a case–cohort analysis approach. Dietary acrylamide intake of subcohort members (n = 1449) and ER+ breast cancer cases (n = 844) was assessed with a food frequency questionnaire. We genotyped single nucleotide polymorphisms (SNPs) in genes in acrylamide metabolism, sex steroid systems, oxidative stress and DNA repair. Multiplicative interaction between acrylamide intake and SNPs was assessed with Cox proportional hazards analysis, based on 20.3 years of follow-up.
Results
Unexpectedly, there was a statistically non-significant inverse association between acrylamide and ER+ breast cancer risk among all women but with no clear dose–response relationship, and no association among never smokers. Among the results for 57 SNPs and 2 gene deletions, rs1056827 in CYP1B1, rs2959008 and rs7173655 in CYP11A1, the GSTT1 gene deletion, and rs1052133 in hOGG1 showed a statistically significant interaction with acrylamide intake for ER+ breast cancer risk.
Conclusions
This study did not provide evidence for a positive association between acrylamide intake and ER+ breast cancer risk. If anything, acrylamide was associated with a decreased ER+ breast cancer risk. The interaction with SNPs in CYP1B1 and CYP11A1 suggests that acrylamide may influence ER+ breast cancer risk through sex hormone pathways.
Similar content being viewed by others
Introduction
Acrylamide, a probable human carcinogen (IARC class 2A), was discovered in 2002 in various heat-treated carbohydrate-rich foods, such as cookies, potato crisps, French fries and coffee. Acrylamide is a small hydrophilic compound that is distributed throughout the body with the blood. In theory, it can thus cause cancer everywhere in the body. Acrylamide is a multisite carcinogen in rodents, in which it causes, among other, mammary gland tumors in females [1]. The mechanisms by which acrylamide causes mammary gland tumors in rodents are hypothesized to be genotoxicity and endocrine effects [1]. Since 2002, a few epidemiological studies have investigated the impact of dietary acrylamide intake on human cancer risks. The results of these studies for breast cancer are inconsistent. A recent meta-analysis did not show an increased risk of breast cancer overall, with a relative risk in the highest category of intake versus the lowest of 0.96 (95% CI 0.91–1.02) [2]. However, our previous analysis in the Netherlands Cohort Study [3] and a Danish study using acrylamide hemoglobin adducts as a marker of internal acrylamide exposure [4] gave some indications for a positive association between acrylamide intake and estrogen receptor-positive (ER+) breast cancer risk. Nevertheless, the above-mentioned meta-analysis did not show an increased risk of this type of breast cancer associated with dietary acrylamide intake either [RR 0.98 (95% CI 0.89–1.08)] [2].
In the present study, we investigated whether genetic make-up modifies the association between acrylamide and ER+ breast cancer risk, thereby contributing to evidence on acrylamide’s possible mechanism of action and on the causality of the observed associations. We focused on ER+ breast cancer because of the hypothesized effect of acrylamide on sex hormones and the fact that two studies observed an increased acrylamide-associated risk with this subtype of breast cancer. For ER+ breast cancers, the involvement of sex hormones in their etiology is probably stronger than for estrogen receptor-negative breast cancers [5]. We selected SNPs in candidate genes in acrylamide metabolism and in mechanisms through which acrylamide is hypothesized to cause cancer: mechanisms involving sex hormones, oxidative stress, and DNA damage caused by glycidamide, acrylamide’s genotoxic metabolite [6]. Previously, we investigated acrylamide intake and gene interactions for endometrial and ovarian cancer risk, and we observed indications for interaction between acrylamide intake and SNPs in among other cytochrome P450, family 2, subfamily E, polypeptide 1 (CYP2E1) and the deletions of the genes glutathione s-transferase M1 and T1 (GSTM1 and GSTT1) [7, 8].
Subjects and methods
Study cohort, cases and follow-up
The Netherlands Cohort Study on diet and cancer started in September 1986 with the inclusion of 62,573 women that were 55–69 years of age, all presumed to be post-menopausal. Data on dietary habits and other risk factors were collected by means of a self-administered questionnaire at baseline in 1986. In addition to the questionnaire, approximately 75% of the participants sent in toenail clippings, as requested.
Following the case–cohort approach, ER+ breast cancer cases were enumerated for the entire cohort, while the accumulated person-years for the entire cohort were estimated from a subcohort of 2589 women randomly sampled from the entire cohort at baseline. Since the start of the study, the subcohort has been followed up regularly for vital status information. Incident cancer cases in the total cohort have been detected by computerized record linkages to the Netherlands Cancer Registry, the Netherlands Pathology Registry and the causes of death registry. Further details on the design of the study and methods of follow-up are presented elsewhere [9,10,11,12].
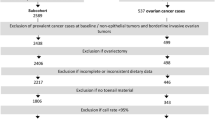
After 20.3 years of follow-up, from September 1986 to December 2006, and after exclusion of cohort members who reported a diagnosis of cancer (except skin cancer) at baseline, there were 1620 microscopically confirmed invasive ER+ primary carcinomas of the breast ([ICD-O]-3: C50). Information on estrogen receptor status was obtained from the National Cancer Registry and the Dutch Pathology Registry and was assessed by either immunohistochemistry or biochemical assay. Cases and subcohort members were excluded from analysis if their dietary data were incomplete or inconsistent, if they had not sent in toenail clippings, and if they had no or inferior (call rate < 95%) data on SNPs. Figure 1 shows the selection and exclusion steps that resulted in the numbers of cases and subcohort members that were available for analysis.
Acrylamide intake assessment
A valid and reproducible food frequency questionnaire with questions on 150 food items was used for estimating dietary habits [11, 12]. Dietary acrylamide intake was estimated from the mean acrylamide level of foods on the Dutch market, and the frequency of consumption and portion size of the foods, as described in detail elsewhere [13].
Selection of genes and SNPs
The selection of genes focused on genes involved in (1) acrylamide metabolism and (2) the most often hypothesized mechanisms of acrylamide-induced carcinogenesis [6]: (2a) sex hormonal effect (involving sex hormone synthesis/metabolism or sex hormone nuclear receptors), (2b) oxidative stress and (2c) genotoxicity (DNA repair), or (2d) SNPs in genes that have clearly been shown in the literature to play a role in carcinogenesis. A detailed description of the selection of genes and SNPs is presented elsewhere [7].
In the end, we genotyped 6 SNPs to determine GSTM1 and GSTT1 deletions (3 SNPs each) and 60 SNPs in other genes, see Supplemental Table 1.
DNA isolation and genotyping
DNA was isolated from 15 mg of toenail clippings, following the protocol developed by Cline et al. [14], in an optimised form [15]. Genotyping was performed by Agena in Hamburg, on the MassARRAY platform using the iPLEX TM assay [16] This method has been successfully used before to genotype DNA from toenails [7, 15, 17, 18].
Supplemental Table 2 shows the 60 SNPs with their location, call frequencies, and HWE p value. 3 out of the 60 SNPs that were genotyped had a call rate < 80% and were not included in the analyses. 6 SNPs out of the remaining 57 SNPs did not adhere to Hardy–Weinberg equilibrium (HWE) (p < 0.05). With regard to the SNPs selected to represent the GSTM1 deletion, rs10857795 was not called in 36%, rs200184852 in 42% and rs74837985 in only 2% of the subcohort. The latter value appears to be due to genotyping error. Therefore, we decided to base the assessment of the absence/presence of the GSTM1 gene only on rs10857795 and rs200184852. 31% of the subcohort had a missing value for both rs10857795 and rs200184852. With regard to GSTT1, rs2844008 was not called in 58%, rs4630 in 16%, and rs140309 in 11% of the subcohort. 8% of the subcohort had a missing value for all 3 GSTT1 SNPs.
5% of the samples (n = 190) were duplicate samples in order to check the reproducibility of genotyping, which was > 99%. We excluded samples with a call rate < 95% (113 breast cancer cases, 93 subcohort members).
Statistical analysis
Hazard rate ratios (HRs) and 95% confidence intervals were obtained through Cox proportional hazards regression with STATA software (package 13), using the robust Huber–White sandwich estimator to account for additional variance introduced by sampling from the cohort. We checked the proportional hazards assumption using scaled Schoenfeld residuals.
Covariables selected for inclusion in the Cox proportional hazards analysis models were selected based on the literature: age, body mass index, height, age at menarche, age at menopause, age at first childbirth, parity, ever use of oral contraceptives, ever use of postmenopausal hormones, history of benign breast disease, family history of breast cancer and energy intake. Smoking status, the duration of smoking and the number of cigarettes per day were included in the model, because cigarette smoke contains acrylamide. Smokers have on average four times higher levels of acrylamide-hemoglobin adducts than non-smokers [19, 20]. To eliminate the influence of acrylamide through smoking, we performed subgroup analyses restricted to never smokers. In addition, we checked the confounding potential of various dietary factors, e.g., alcohol, fibre, glycaemic index, but none changed the hazard ratio of acrylamide by more than 10%. The main associations between SNPs and ER+ breast cancer risk were only adjusted for age.
Multiplicative interaction between acrylamide intake and SNPs was tested using product terms of the continuous acrylamide intake variable and genotype. For statistical power reasons, we used a dominant genetic model for all SNPs. Tests for acrylamide dose–response trends in strata of the genotypes were performed by fitting the mean acrylamide intake in the tertiles as a continuous variable.
We applied the false discovery rate method developed by Benjamini–Hochberg to adjust for multiple testing [21] with the expected proportion of false positives set at 20% [22, 23].
Two-sided p values are reported throughout this paper.
Results
Table 1 shows the characteristics of the subcohort and ER+ breast cancer cases at baseline. Cases reported to have fewer children and to more often be nulliparous than subcohort members. Cases reported more often to be current smokers, to have smoked more cigarettes and for a longer duration, and to have drunk more alcohol than subcohort members. They reported more often to have ever used postmenopausal hormone treatment and less often to have ever used oral contraceptives. Furthermore, cases reported more often to have a personal history of benign breast disease and family history of breast cancer.
There was a statistically non-significant inverse association between acrylamide and ER+ breast cancer risk after 20.3 years of follow-up in all women (HR of highest versus the lowest quintile of intake: 0.85 (95% CI 0.66–1.09) and 0.94 (0.88–1.00) per 10 µg/day increment of intake) but with no clear dose–response relationship. There was no association in never-smoking women (HR of highest versus the lowest quintile of intake: 1.18 (95% CI 0.85–1.64) and 1.02 (0.93–1.11) per 10 µg/day increment of intake) (Table 2).
Table 3 presents the SNPs showing a trend for ER+ breast cancer over the number of variant alleles after 20.3 years of follow-up. None of the SNPs was statistically significantly associated with ER+ breast cancer risk after adjustment for multiple comparisons. There were some nominally statistically significant interactions. There was a statistically non-significant decrease in risk with an increasing number of variant alleles for rs2070959 in UDP glucuronosyltransferase family 1 member A complex (UGT1A) (p trend = 0.08), rs4919682 and rs4919687 in cytochrome P450, family 17, subfamily A, polypeptide 1 (CYP17A1) (p trend = 0.07 and 0.05, respectively), rs915906 in CYP2E1 (p trend = 0.09), and rs3219489 in human MutY homolog (hMYH) (p trend = 0.07).
Table 4 shows the interactions between SNPs and acrylamide intake that remained statistically significant after adjustment for multiple comparisons.
The homozygous deletion of GSTT1, when represented by rs140309, showed a statistically significant interaction with acrylamide intake (p interaction = 0.01) and this interaction remained statistically significant after adjustment for multiple testing (Benjamini–Hochberg adjusted p value 0.19). The same interaction was observed for never smokers. Women with a homozygous deletion of the GSTT1 gene were at a statistically significantly decreased acrylamide-associated risk of ER+ breast cancer [HR 0.35 (95% CI 0.15–0.83) among all women and HR 0.16 (95% CI 0.04–0.68) among never-smoking women in the highest tertile of acrylamide intake versus the lowest], while there was no association with acrylamide intake among women with at least 1 copy of the gene.
There were four more interactions that remained statistically significant after adjustment for multiple testing, with the following SNPs: rs1056827 in cytochrome P450, family 1, subfamily B, polypeptide 1 (CYP1B1) (Benjamini–Hochberg adjusted p value 0.18), rs2959008 and rs7173655 (R2 0.79, D′ 0.92) in cytochrome P450, family 11, subfamily A, polypeptide 1 (CYP11A1) (Benjamini–Hochberg adjusted p value 0.19 for both SNPs), and rs1052133 in human 8-oxo-7,8-dihydroguanine DNA glycosylase 1 (hOGG1) (Benjamini–Hochberg adjusted p value 0.19). Women homozygous for the wild-type allele of CYP1B1 were at a statistically significantly decreased acrylamide-associated risk of ER+ breast cancer, while women with at least 1 variant allele were not. Among never smokers, this pattern was not seen, although the interaction was nominally statistically significant (p interaction = 0.03). In this subgroup, there was a statistically non-significant increase in risk in women with at least one variant allele, while there was no association between acrylamide and risk in women with two wild-type alleles. The two SNPs in CYP11A1 both showed that women homozygous for the wild-type allele were at a decreased acrylamide-associated risk while there was no association for women with at least one variant allele. This was seen both among all women and among never smokers. Only women with one or two variant alleles of rs1052133 in hOGG1 were at a decreased acrylamide-associated risk of ER+ breast cancer. Women who were homozygous wild types did not show an association between acrylamide and ER+ breast cancer. The effect modification was less clear among never smokers.
In Table 5, we show interactions with SNPs in (other) genes involved in acrylamide metabolism that are interesting because they have a higher a priori probability of modifying the association between acrylamide and cancer risk than the other selected SNPs. Rs915906, rs2480258, and rs6413432 in CYP2E1 did not show an interaction with acrylamide intake among all women, nor among never-smoking women. There was also no interaction between the deletion of GSTM1 and acrylamide, or SNPs in other acrylamide-metabolizing genes.
Supplemental Table 3 shows the nominally statistically significant interactions that did not withstand adjustment for multiple testing, namely: rs1800566 in NQO1 and rs6838248 in SLC7A11 specifically among never smokers.
Finally, there were some clear differences in the association with acrylamide between genotypes without a statistically significant interaction, namely for: rs2070959 in UGT1A, rs11252859 in AKR1C1, rs11252887 in AKR1C2, rs1280350 in MGC12965, rs1042157 and rs6839 in SULT1A1, rs737865 in COMT, rs10432782 in SOD1, rs4880 and rs5746136 in SOD2, rs1047303 in the HSD3B1/B2 gene cluster, rs6259 in SHBG, rs6759180 in RRM2, and rs2228001 in XPC (Supplemental Table 3).
Discussion
As far as we know, this is the first study to analyze acrylamide-gene interactions for breast cancer risk. We observed interactions between acrylamide intake and rs1056827 in CYP1B1, rs2959008 and rs7173655 in CYP11A1, the GSTT1 gene deletion, and rs1052133 in hOGG1. These interactions remained statistically significant after adjustment for multiple testing.
Contrary to what we found in a previous analysis for ER+ breast cancer albeit not statistically significant [3], acrylamide intake was not positively associated with ER+ breast cancer risk among never smokers in the current analysis. In addition, when we restricted our analyses to 13.3 years of follow-up (as in the previous analysis), acrylamide intake was not positively associated with ER+ breast cancer risk. The explanation for this discrepancy is probably that different case sets were used in the analyses. In the previous analyses, cases were derived from the Dutch Pathology Registry and four regional Dutch cancer registries because only those 4 routinely recorded information on estrogen receptor status at that time. Cases for the current analysis originated from all nine regional Dutch cancer registries, the Dutch Pathology Registry and the causes of death registry, and so there were more cases in the present analysis. In the previous analysis, the percentage of cases with missing info on estrogen receptor status was quite high (57%) and we checked whether cases with known estrogen receptor status differed from cases with unknown receptor status with regard to tumor and other characteristics, such as BMI and age. This was not the case but it is still possible that selection of a specific subgroup of ER+ cases occurred and that the positive association between acrylamide intake and ER+ breast cancer risk was restricted to this group. Among all women in the current analysis, there was a tendency towards an inverse association.
Glycidamide (the epoxide metabolite of acrylamide formed through metabolism by CYP2E1) is often thought to be responsible for acrylamide-induced carcinogenesis due to genotoxicity (mutagenicity and/or clastogenicity [24]). Studying the modifying effect of SNPs in CYP2E1 on the association between acrylamide and cancer risk may thus contribute important information on the causality of the association. We observed no interaction between 3 SNPS in CYP2E1 and acrylamide intake for ER+ breast cancer risk and there were no clear differences in the risk between the genotypes, contrary to what was observed for endometrial [7] and ovarian cancer [8]. We have no clear explanation for this inconsistency but an explanation could be that there is no association between acrylamide intake and breast cancer risk or that for breast cancer, acrylamide and glycidamide are (roughly) equally responsible for the carcinogenic effect. The three studied CYP2E1 SNPs are in the intronic region of the gene and there is no clear information about their functionality but the wild-type allele of rs6413432 has been shown to be associated with an increased risk of prostate cancer [25] and other cancers such as lung cancer [26].
We observed that women with a homozygous deletion of GSTT1 (deletion represented by rs140309) were at a decreased acrylamide-associated risk of ER+ breast cancer but the number of cases with a homozygous GSTT1 deletion was rather small (n = 87). In contrast, we previously observed women with at least one copy of the GSTT1 gene to be at an increased acrylamide-associated risk of endometrial and ovarian cancer [7, 8]. There was no clear difference in the association between acrylamide intake and ER+ breast cancer risk between the genotypes of GSTM1.
We observed a statistically significant interaction between acrylamide intake and rs1056827 in CYP1B1. Women who were homozygous wild types for this allele were at a decreased risk of acrylamide-associated ER+ breast cancer. In never smokers, the interaction was less clear. CYP1B1 is a phase I biotransformation enzyme involved in the metabolism of various exogenous and endogenous compounds and is mainly expressed in endocrine tissues like the endometrium, ovarium and breast. CYP1B1 converts estrogens to hydroxy metabolites (catechol estrogens) which are potent estrogens and furthermore CYP1B1 oxidizes catechol estrogens to chemically reactive semiquinone and quinone intermediates that can bind to DNA and cause mutations. Exposure of mouse spermatocytes to acrylamide and glycidamide led to increased CYP1B1 expression [27], while glycidamide exposure led to decreased CYP1B1 expression in human epithelial cells [28]. Possible explanations for this discrepancy are species, cell or dose differences. The variant allele of rs1056827 has been shown to have increased enzyme activity and to be associated with an increased breast cancer risk [29]. Due to the scarcity of literature and the inconsistency in the relationship between acrylamide and CYP1B1 activity, it is currently impossible to say whether the observed interaction is biologically plausible.
Acrylamide interacted statistically significantly with 2 SNPs in CYP11A1: rs2959008 and rs7173655; acrylamide intake was associated with a decreased ER+ breast cancer risk in women who were homozygous for the wild type of these alleles. CYP11A1 is involved in the formation of pregnenolone from cholesterol, the first and rate-limiting step in steroid hormone synthesis. Both CYP11A1 SNPs are in the intronic region of the gene and there is no information available about their functionality. The variant allele of rs2959008 was associated with a decreased breast cancer risk in Han Chinese [30]. There is no literature on rs7173655 and breast cancer risk but the variant allele was associated with an increased endometrial cancer risk [31]. Acrylamide exposure of male Fischer 344 rats led to increased expression of CYP11A1 in reproductive tissues [32] but to decreased CYP11A1 expression in testis tissue of male Sprague–Dawley rats [33]. This discrepancy may be due to differences in rat strains or doses of acrylamide, or both. Thus, again due to the scarcity and inconsistency of data, it is not currently possible to judge the biological plausibility of the interaction between acrylamide and these CYP11A1 SNPs.
Progesterone opposes the proliferative effect of estrogens in the endometrium and ovaries while it is thought to have proliferative effects in mammary tissue [34]. A mechanism by which acrylamide may increase risks of endometrial and ovarian cancer and decrease the risk of ER+ breast cancer is through an effect on progesterone. However, this is highly speculative, also due to the fact that we did not see interaction between acrylamide and the CYP2E1 SNPs which leaves the possibility that there may not be a true association between acrylamide intake and breast cancer risk. If true, the interactions between acrylamide and HSD3B SNPs for ovarian cancer [8] and between acrylamide and the CYP1B1 and CYP11A1 SNPs for breast cancer in the current study give some indications that acrylamide may interfere with progesterone metabolism. However, there was no association between acrylamide intake and progesterone in a cross-sectional study in premenopausal women [35]. In animals, acrylamide has repeatedly been shown to decrease progesterone levels [36,37,38]. Nevertheless, we strongly encourage more research on the possible effect of acrylamide on progesterone metabolism in humans.
Only women with variant alleles of rs1052133 in hOGG1 showed an inverse association between acrylamide intake and ER+ breast cancer risk. The hOGG1 gene is part of the base excision DNA repair pathway, responsible for the excision of 8-oxoguanine (8-oxoG), a mutagenic DNA base byproduct of reactive oxygen species. Although the variant allele is hypothesized to have decreased enzyme activity [39, 40], a recent meta-analysis showed that it is associated with a decreased risk of breast cancer among Europeans [41], while another meta-analysis did not show an association [42]. Because of these apparent contradictions, it is currently impossible to speculate about the possible mechanism by which this SNP could modify the association between acrylamide intake and ER+ breast cancer risk.
There were two other nominally statistically significant interactions between acrylamide intake and other SNPs: rs1800566 in NQO1 and rs6838248 in SLC7A11. Additionally, there were some clear differences in the association with acrylamide between genotypes without a statistically significant interaction, for: rs2070959 in UGT1A, rs11252859 in AKR1C1, rs11252887 in AKR1C2, rs1280350 in MGC12965, rs1042157 and rs6839 in SULT1A1, rs737865 in COMT, rs10432782 in SOD1, rs4880 and rs5746136 in SOD2, rs1047303 in the HSD3B1/B2 gene cluster, rs6259 in SHBG, rs6759180 in RRM2, and rs2228001 in XPC. For all these SNPs it is even more important that the interaction between acrylamide intake and these SNPs is corroborated in other studies to be able to judge whether our findings represent true interactions or not.
This study has some limitations. Acrylamide levels vary considerably within foods due to processing and varieties used. Despite the large variation in acrylamide levels within foods, acrylamide intake as assessed by food frequency questionnaires and acrylamide to hemoglobin adducts (biomarker for exposure) have been shown to correlate moderately in several studies, e.g. [43, 44]. Thus, the food frequency questionnaire is able to estimate the rank order of acrylamide intake among study populations. In addition, we correlated the assessed acrylamide intake (based on mean acrylamide levels per food) and the measured acrylamide content of 24-hour Dutch duplicate diets, for which the participants had written down exactly what and how much they ate and drank. The correlation was very high (r = 0.82, p < 0.001) [45]. To conclude, assessing acrylamide intake through food frequency questionnaires is not perfect and entails some random measurement error, which pushes the point estimate towards the null, but it is useful for studying the link between acrylamide intake and cancer risk. Data on diet and covariables obtained from the questionnaire were collected only once, at baseline. Some of the characteristics (e.g., diet, BMI) will certainly have changed over time after baseline. One of the reasons to select an elderly population for the study was that older people tend to have more stable dietary habits. The changes that have occurred despite this will have resulted in random measurement error, pushing the point estimate of the hazard ratio towards the null.
Some of the nominally statistically significant interactions that we observed are, without a doubt, chance findings. However, it is of interest that some of the genes that we observed to interact with acrylamide for ER+ breast cancer risk or that showed clear differences in risk between the genotypes also did so for endometrial [7] and ovarian cancer [8]: GSTT1, AKR1C1, NQO1, the HSD3B1/B2 gene cluster, XPC, and MGC12965. These genes therefore deserve attention in future studies.
The strengths of this study are the complete follow-up and its prospective nature.
In conclusion, we did not observe a positive association between dietary acrylamide intake and ER+ breast cancer risk. Unexpectedly, our results gave some indications for an inverse association. Unlike for endometrial and ovarian cancer, there was no interaction between acrylamide intake and CYP2E1 SNPs for ER+ breast cancer risk. After adjustment for multiple testing, this study showed statistically significant interactions between rs1056827 in CYP1B1, rs2959008 and rs7173655 in CYP11A1, the deletion of GSTT1, and rs1052133 in hOGG1 and acrylamide intake for ER+ breast cancer risk. Based on this study and analyses for endometrial and ovarian cancer, we recommend follow-up of interactions between acrylamide intake and genetic polymorphisms in CYP1B1, CYP11A1, the HSD3B1/B2 gene cluster, CYP2E1, GSTs, hOGG1, AKR1C1, NQO1, GPX1, XPC and MGC12965, and additional research on the possible effect of acrylamide on progesterone metabolism in humans.
References
Maier A, Kohrman-Vincent M, Hertzberg R, Allen B, Haber LT, Dourson M (2012) Critical review of dose-response options for F344 rat mammary tumors for acrylamide - additional insights based on mode of action. Food Chem Toxicol 50(5):1763–1775
Pelucchi C, Bosetti C, Galeone C, La Vecchia C (2015) Dietary acrylamide and cancer risk: an updated meta-analysis. Int J Cancer 136(12):2912–2922
Pedersen GS, Hogervorst JG, Schouten LJ, Konings EJ, Goldbohm RA, van den Brandt PA (2010) Dietary acrylamide intake and estrogen and progesterone receptor-defined postmenopausal breast cancer risk. Breast Cancer Res Treat 122(1):199–210
Olesen PT, Olsen A, Frandsen H, Frederiksen K, Overvad K, Tjonneland A (2008) Acrylamide exposure and incidence of breast cancer among postmenopausal women in the Danish Diet, Cancer and Health Study. Int J Cancer 122(9):2094–2100
Althuis MD, Fergenbaum JH, Garcia-Closas M, Brinton LA, Madigan MP, Sherman ME (2004) Etiology of hormone receptor-defined breast cancer: a systematic review of the literature. Cancer Epidemiol Biomarkers Prev 13(10):1558–1568
Besaratinia A, Pfeifer GP (2007) A review of mechanisms of acrylamide carcinogenicity. Carcinogenesis 28(3):519–528
Hogervorst JG, van den Brandt PA, Godschalk RW, van Schooten FJ, Schouten LJ (2016) The influence of single nucleotide polymorphisms on the association between dietary acrylamide intake and endometrial cancer risk. Sci Rep 6:34902
Hogervorst JG, van den Brandt PA, Godschalk RW, van Schooten FJ, Schouten LJ (2017) Interactions between dietary acrylamide intake and genes for ovarian cancer risk. Eur J Epidemiol 32(5):431–441
van den Brandt PA, Goldbohm RA, van ‘t Veer P, Volovics A, Hermus RJ, Sturmans F (1990) A large-scale prospective cohort study on diet and cancer in The Netherlands. J Clin Epidemiol 43(3):285–295
van den Brandt PA, Schouten LJ, Goldbohm RA, Dorant E, Hunen PM (1990) Development of a record linkage protocol for use in the Dutch Cancer Registry for Epidemiological Research. Int J Epidemiol 19(3):553–558
Goldbohm RA, van den Brandt PA, Brants HA, van’t Veer P, Al M, Sturmans F, Hermus RJ (1994) Validation of a dietary questionnaire used in a large-scale prospective cohort study on diet and cancer. Eur J Clin Nutr 48(4):253–265
Goldbohm RA, van ‘t Veer P, van den Brandt PA, van ‘t Hof MA, Brants HA, Sturmans F, Hermus RJ (1995) Reproducibility of a food frequency questionnaire and stability of dietary habits determined from five annually repeated measurements. Eur J Clin Nutr 49(6):420–429
Hogervorst JG, Schouten LJ, Konings EJ, Goldbohm RA, van den Brandt PA (2007) A prospective study of dietary acrylamide intake and the risk of endometrial, ovarian, and breast cancer. Cancer Epidemiol Biomarkers Prev 16(11):2304–2313
Cline RE, Laurent NM, Foran DR (2003) The fingernails of Mary Sullivan: developing reliable methods for selectively isolating endogenous and exogenous DNA from evidence. J Forensic Sci 48(2):328–333
Hogervorst JG, Godschalk RW, van den Brandt PA, Weijenberg MP, Verhage BA, Jonkers L, Goessens J, Simons CC, Vermeesch JR, van Schooten FJ et al (2014) DNA from nails for genetic analyses in large-scale epidemiologic studies. Cancer Epidemiol Biomarkers Prev 23(12):2703–2712
Gabriel S, Ziaugra L, Tabbaa D (2009) SNP genotyping using the Sequenom MassARRAY iPLEX platform. Curr Protoc Hum Genet Chap 2:Unit 2 12
Geybels MS, van den Brandt PA, Schouten LJ, van Schooten FJ, van Breda SG, Rayman MP, Green FR, Verhage BA (2014) Selenoprotein gene variants, toenail selenium levels, and risk for advanced prostate cancer. J Nat Cancer Inst 106(3):dju003
Deckers IA, van den Brandt PA, van Engeland M, van Schooten FJ, Godschalk RW, Keszei AP, Schouten LJ (2015) Polymorphisms in genes of the renin-angiotensin-aldosterone system and renal cell cancer risk: Interplay with hypertension and intakes of sodium, potassium and fluid. Int J Cancer 136(5):1104–1116
Schettgen T, Rossbach B, Kutting B, Letzel S, Drexler H, Angerer J (2004) Determination of haemoglobin adducts of acrylamide and glycidamide in smoking and non-smoking persons of the general population. Int J Hyg Environ Health 207(6):531–539
Bergmark E (1997) Hemoglobin adducts of acrylamide and acrylonitrile in laboratory workers, smokers and nonsmokers. Chem Res Toxicol 10(1):78–84
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc 57:289–300
Geybels MS, van den Brandt PA, van Schooten FJ, Verhage BA (2015) Oxidative stress-related genetic variants, pro- and antioxidant intake and status, and advanced prostate cancer risk. Cancer Epidemiol Biomarkers Prev 24(1):178–186
Kim C, Zheng T, Lan Q, Chen Y, Foss F, Chen X, Holford T, Leaderer B, Boyle P, Chanock SJ et al (2012) Genetic polymorphisms in oxidative stress pathway genes and modification of BMI and risk of non-Hodgkin lymphoma. Cancer Epidemiol Biomarkers Prev 21(5):866–868
Wang RS, McDaniel LP, Manjanatha MG, Shelton SD, Doerge DR, Mei N (2010) Mutagenicity of acrylamide and glycidamide in the testes of big blue mice. Toxicol Sci 117(1):72–80
Ferreira PM, Medeiros R, Vasconcelos A, Costa S, Pinto D, Morais A, Oliveira J, Lopes C (2003) Association between CYP2E1 polymorphisms and susceptibility to prostate cancer. Eur J Cancer Prev 12(3):205–211
Su XL, Bin B, Cui HW, Ran MR (2011) Cytochrome P450 2E1 RsaI/PstI and DraI polymorphisms are risk factors for lung cancer in mongolian and han population in inner Mongolia. Chin J Cancer Res 23(2):107–111
Nixon BJ, Katen AL, Stanger SJ, Schjenken JE, Nixon B, Roman SD (2014) Mouse spermatocytes express CYP2E1 and respond to acrylamide exposure. PloS one 9(5):e94904
Clement FC, Dip R, Naegeli H (2007) Expression profile of human cells in culture exposed to glycidamide, a reactive metabolite of the heat-induced food carcinogen acrylamide. Toxicology 240(1–2):111–124
Reding KW, Weiss NS, Chen C, Li CI, Carlson CS, Wilkerson HW, Farin FM, Thummel KE, Daling JR, Malone KE (2009) Genetic polymorphisms in the catechol estrogen metabolism pathway and breast cancer risk. Cancer Epidemiol Biomarkers Prev 18(5):1461–1467
Sun M, Yang X, Ye C, Xu W, Yao G, Chen J, Li M (2012) Risk-association of CYP11A1 polymorphisms and breast cancer among Han Chinese women in Southern China. Int J Mol Sci 13(4):4896–4905
Terry K, McGrath M, Lee IM, Buring J, De Vivo I (2010) Genetic variation in CYP11A1 and StAR in relation to endometrial cancer risk. Gynecol Oncol 117(2):255–259
Camacho L, Latendresse JR, Muskhelishvili L, Patton R, Bowyer JF, Thomas M, Doerge DR (2012) Effects of acrylamide exposure on serum hormones, gene expression, cell proliferation, and histopathology in male reproductive tissues of Fischer 344 rats. Toxicol Lett 211(2):135–143
Yang HJ, Lee SH, Jin Y, Choi JH, Han DU, Chae C, Lee MH, Han CH (2005) Toxicological effects of acrylamide on rat testicular gene expression profile. Reprod Toxicol 19(4):527–534
Diep CH, Daniel AR, Mauro LJ, Knutson TP, Lange CA (2015) Progesterone action in breast, uterine, and ovarian cancers. J Mol Endocrinol 54(2):R31-53
Hogervorst JG, Fortner RT, Mucci LA, Tworoger SS, Eliassen AH, Hankinson SE, Wilson KM (2013) Associations between dietary acrylamide intake and plasma sex hormone levels. Cancer Epidemiol Biomarkers Prev 22(11):2024–2036
Lebda M, Gad S, Gaafar H (2014) Effects of lipoic acid on acrylamide induced testicular damage. Mater Sociomed 26(3):208–212
Shuming C, Jilin F, Xichun Z (2009) The moderating role of dark soy sauce to acrylamide-induced oxidative stress and neurophysiological perturbations in rats. Toxicol Mech Methods 19(6–7):434–440
Wei Q, Li J, Li X, Zhang L, Shi F (2014) Reproductive toxicity in acrylamide-treated female mice. Reprod Toxicol 46:121–128
Kohno T, Shinmura K, Tosaka M, Tani M, Kim SR, Sugimura H, Nohmi T, Kasai H, Yokota J (1998) Genetic polymorphisms and alternative splicing of the hOGG1 gene, that is involved in the repair of 8-hydroxyguanine in damaged DNA. Oncogene 16(25):3219–3225
Lee AJ, Hodges NJ, Chipman JK (2005) Interindividual variability in response to sodium dichromate-induced oxidative DNA damage: role of the Ser326Cys polymorphism in the DNA-repair protein of 8-oxo-7,8-dihydro-2′-deoxyguanosine DNA glycosylase 1. Cancer Epidemiol Biomarkers Prev 14(2):497–505
Yuan Y, Chen F, Zhao GH, Liu J, Zhang HX, Hu XS (2007) A comparative study of acrylamide formation induced by microwave and conventional heating methods. J Food Sci 72(4):C212-216
Gu D, Wang M, Zhang Z, Chen J (2010) Lack of association between the hOGG1 Ser326Cys polymorphism and breast cancer risk: evidence from 11 case-control studies. Breast Cancer Res Treat 122(2):527–531
Wilson KM, Bälter K, Adami HO, Grönberg H, Vikström AC, Paulsson B, Törnqvist M, Mucci LA (2009) Acrylamide exposure measured by food frequency questionnaire and hemoglobin adduct levels and prostate cancer risk in the Cancer of the Prostate in Sweden Study. Int J Cancer 124(10):2384–2390
Wilson KM, Vesper HW, Tocco P, Sampson L, Rosén J, Hellenäs KE, Törnqvist M, Willett WC (2009) Validation of a food frequency questionnaire measurement of dietary acrylamide intake using hemoglobin adducts of acrylamide and glycidamide. Cancer Causes Control 20(3):269–278
Konings EJ, Hogervorst JG, van Rooij L, Schouten LJ, Sizoo EA, van Egmond HP, Goldbohm RA, van den Brandt PA (2010) Validation of a database on acrylamide for use in epidemiological studies. Eur J Clin Nutr 64(5):534–540
Acknowledgements
This study was funded by the Dutch Cancer Society (KWF), Grant number: UM 2011–5123. Janneke Hogervorst is a postdoctoral research fellow from the Research Foundation—Flanders (FWO), no. 12J9516N. The authors thank the study participants, the Netherlands Cancer Registry, the Dutch Pathology Registry, and the Biobank of the Maastricht University Medical Center. We thank Dr. Sandra Bausch as initiator of the NLCS study, together with Prof. Piet van den Brandt. We also thank Sacha van de Crommert, Jolanda Nelissen, Conny de Zwart, Ellen Dutman, Henny Brants, and Annemie Pisters for their assistance with data entry or data management, Harry van Montfort for programming assistance, and Simone van Breda, Stijn Lumeij, Kristien Lemmens, Joy Goessens, and Leonie Jonkers for technical assistance with DNA isolation and genotyping.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare. Leo Schouten was compensated for being on an expert panel of the European Food Safety Authority that contributed to the 2015 risk assessment on acrylamide.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Hogervorst, J.G.F., van den Brandt, P.A., Godschalk, R.W.L. et al. Interaction between dietary acrylamide intake and genetic variants for estrogen receptor-positive breast cancer risk. Eur J Nutr 58, 1033–1045 (2019). https://doi.org/10.1007/s00394-018-1619-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-018-1619-z