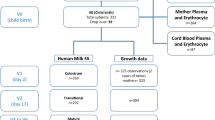
Abstract
Purpose
Essential fatty acids play a critical role in the growth and development of infants, but little is known about the fatty acid status of populations in low-income countries. The objective was to describe the fatty acid composition of red blood cells (RBC) in breastfeed Nepali infants and a subsample of their mothers and to identify the main sources of fatty acids in the mother’s diet, as well as the fatty acid composition of breast milk.
Methods
RBC fatty acid composition was analyzed in a random sample of 303 infants and 72 mother, along with 68 breastmilk samples. Fatty acid profiles of the most important dietary fat sources were analyzed. Information on mother’s diet and intake of fat was collected by three 24-h dietary recalls.
Results
In infant RBC’s, docosahexaenoic acid (DHA) was the main n-3 fatty acid, and arachidonic acid (AA) was the major n-6 fatty acid. Total n-6 PUFA was three times higher than total n-3 PUFA. Height-for-age (HAZ) was positively associated with DHA status and AA status in multivariable models. The concentration of all fatty acids was higher in children, compared to mothers, except Total n-6 PUFA and Linoleic acid (LA) where no differences were found. The mother’s energy intake from fat was 13% and cooking oil (sesame, mustard, soybean or sunflower oil) contributed 52% of the fat intake.
Conclusions
RBC-DHA levels in both infants and mother was unexpected high taking into account few dietary DHA sources and the low DHA concentrations in breastmilk.

Similar content being viewed by others
References
Huffman SL, Harika RK, Eilander A, and Osendarp SJ (2011) Essential fats: how do they affect growth and development of infants and young children in developing countries? A literature review. Matern Child Nutr. 7(Suppl 3):44–65
Lauritzen L, Carlson SE (2011) Maternal fatty acid status during pregnancy and lactation and relation to newborn and infant status. Matern Child Nutr 7:41–58
Makrides M, Collins CT, Gibson RA (2011) Impact of fatty acid status on growth and neurobehavioural development in humans. Matern Child Nutr 7:80–88
Michaelsen KF, Dewey KG, Perez-Exposito AB, Nurhasan M, Lauritzen L, Roos N (2011) Food sources and intake of n-6 and n-3 fatty acids in low-income countries with emphasis on infants, young children (6–24 months), and pregnant and lactating women. Matern Child Nutr 7:124–140
Brenna JT (2002) Efficiency of conversion of alpha-linolenic acid to long chain n-3 fatty acids in man. Curr Opin Clin Nutr Metab Care 5(2):127–132
Pawlosky RJ, Hibbeln JR, Novotny JA, Salem N Jr (2001) Physiological compartmental analysis of alpha-linolenic acid metabolism in adult humans. J Lipid Res 42(8):1257–1265
Vermunt SH, Mensink RP, Simonis AM, and Hornstra G (1999) Effects of age and dietary n-3 fatty acids on the metabolism of [13C]-alpha-linolenic acid. Lipids. 34:S127
Emken EA, Adlof RO, Gulley RM (1994) Dietary linoleic acid influences desaturation and acylation of deuterium-labeled linoleic and linolenic acids in young adult males. Biochim Biophys Acta 1213(3):277–288
Salem N Jr, Pawlosky R, Wegher B, Hibbeln J (1999) In vivo conversion of linoleic acid to arachidonic acid in human adults. Prostaglandins Leukot Essent Fatty Acids 60(5–6):407–410
Uauy R and Castillo C (2003) Lipid requirements of infants: implications for nutrient composition of fortified complementary foods. J Nutr 133(9)2962s–2972s
Innis SM (2007) Fatty acids and early human development. Early Hum Dev 83(12):761–766
Uauy R, Dangour AD (2009) Fat and fatty acid requirements and recommendations for infants of 0–2 years and children of 2–18 years. Ann Nutr Metab 55(1–3):76–96
Smuts CM, Tichelaar HY, van Jaarsveld PJ, Badenhorst CJ, Kruger M, Laubscher R, Mansvelt EP, Benade AJ (1994) The effect of iron fortification on the fatty acid composition of plasma and erythrocyte membranes in primary school children with and without iron-deficiency. Prostaglandins Leukot Essent Fatty Acids 51(4):277–285
Lauritzen L, Hansen HS, Jorgensen MH, Michaelsen KF (2001) The essentiality of long chain n-3 fatty acids in relation to development and function of the brain and retina. Prog Lipid Res 40(1–2):1–94
Briend A, Dewey KG, Reinhart GA (2011) Fatty acid status in early life in low-income countries – overview of the situation, policy and research priorities. Matern Child Nutr 7:141–148
Henjum S, Manger M, Skeie E, Ulak M, Thorne-Lyman AL, Chandyo R, Shrestha PS, Locks L, Ulvik RJ, Fawzi WW, Strand TA (2014) Iron deficiency is uncommon among lactating women in urban Nepal, despite a high risk of inadequate dietary iron intake. Br J Nutr 112(1):132–141
Valentiner-Branth P, Shrestha PS, Chandyo RK, Mathisen M, Basnet S, Bhandari N, Adhikari RK, Sommerfelt H, Strand TA (2010) A randomized controlled trial of the effect of zinc as adjuvant therapy in children 2–35 mo of age with severe or nonsevere pneumonia in Bhaktapur, Nepal. Am J Clin Nutr 91(6):1667–1674
Araujo P, Nguyen TT, Froyland L, Wang J, Kang JX (2008) Evaluation of a rapid method for the quantitative analysis of fatty acids in various matrices. J Chromatogr A 1212(1–2):106–113
Lie O, Lambertsen G (1991) Fatty acid composition of glycerophospholipids in seven tissues of cod (Gadus morhua), determined by combined high-performance liquid chromatography and gas chromatography. J Chromatogr 565(1–2):119–129
Harris WS, Von Schacky C (2004) The Omega-3 Index: a new risk factor for death from coronary heart disease? Prev Med 39(1):212–220
Bokor S, Dumont J, Spinneker A, Gonzalez-Gross M, Nova E, Widhalm K, Moschonis G, Stehle P, Amouyel P, De Henauw S, Molnar D, Moreno LA, Meirhaeghe A, Dallongeville J, Group HS (2010) Single nucleotide polymorphisms in the FADS gene cluster are associated with delta-5 and delta-6 desaturase activities estimated by serum fatty acid ratios. J Lipid Res 51(8):2325–2333
Araujo P, Tilahun E, Breivik JF, Abdulkader BM, Froyland L, Zeng Y (2016) A simple liquid extraction protocol for overcoming the ion suppression of triacylglycerols by phospholipids in liquid chromatography mass spectrometry studies. Talanta 148:463–471
Henjum S, Torheim LE, Thorne-Lyman AL, Chandyo R, Fawzi WW, Shrestha PS, Strand TA (2015) Low dietary diversity and micronutrient adequacy among lactating women in a peri-urban area of Nepal. Public Health Nutr 18(17):3201–3210
Gibson RS, Ferguson EL (2008) An interactive 24-hour recall for assesing the adequacy of iron and zinc intakes in developing countries. HarvestPlus. International Life Sciences Institute, Washington, DC
Haubrock J, Nothlings U, Volatier JL, Dekkers A, Ocke M, Harttig U, Illner AK, Knuppel S, Andersen LF, Boeing H, European Food Consumption Validation C (2011) Estimating usual food intake distributions by using the multiple source method in the EPIC-Potsdam Calibration Study. J Nutr 141(5):914–920
Harttig U, Haubrock J, Knuppel S, Boeing H, Consortium E (2011) The MSM program: web-based statistics package for estimating usual dietary intake using the Multiple Source Method. Eur J Clin Nutr 65(Suppl 1):S87–91
de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J (2007) Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ 85(9):660–667
Hosmer DW, Lemeshow S (2000) Applied Logistic Regression. 2nd edn. Wiley, New York
FAO/WHO (2008), Joint FAO/WHO Experts consultation on Fats and fatty acids in Human Nutrition
Libuda L, Mesch CM, Stimming M, Demmelmair H, Koletzko B, Warschburger P, Blanke K, Reischl E, Kalhoff H, Kersting M (2016) Fatty acid supply with complementary foods and LC-PUFA status in healthy infants: results of a randomised controlled trial. Eur J Nutr 55(4):1633–1644
Markhus MW, Seafood consumption, mental health and infant development (2015) A longitudinal observational study from Norway. Dissertation University of Bergen, Norway
VanderJagt DJ, Arndt CD, Okolo SN, Huang YS, Chuang LT, Glew RH (2000) Fatty acid composition of the milk lipids of Fulani women and the serum phospholipids of their exclusively breast-fed infants. Early Hum Dev 60(2):73–87
Pries AM, Huffman SL, Adhikary I, Upreti SR, Dhungel S, Champeny M, Zehner E (2016) High consumption of commercial food products among children less than 24 months of age and product promotion in Kathmandu Valley, Nepal. Matern Child Nutr. 12 Suppl 2: p. 22–37
Davis BC, Kris-Etherton PM (2003) Achieving optimal essential fatty acid status in vegetarians: current knowledge and practical implications. Am J Clin Nutr 78(3 Suppl):640S–646S
Crawford MA, Wang Y, Forsyth S, Brenna JT (2015) The European Food Safety Authority recommendation for polyunsaturated fatty acid composition of infant formula overrules breast milk, puts infants at risk, and should be revised. Prostaglandins Leukot Essent Fatty Acids 102–103:1–3
Bradbury J (2011) Docosahexaenoic acid (DHA): an ancient nutrient for the modern human brain. Nutrients 3(5):529–554
Cormier H, Rudkowska I, Lemieux S, Couture P, Julien P, Vohl MC (2014) Effects of FADS and ELOVL polymorphisms on indexes of desaturase and elongase activities: results from a pre-post fish oil supplementation. Genes Nutr 9(6):437
Kothapalli K, Ye K, Gadgil MS, Carlson SE, O’Brien KO, Zhang JY, Park HG, Ojukwu K, Zou J, Hyon SS, Joshi KS, Gu Z, Keinan A, Brenna T (2016) Positive selection on a regulatory insertion-deletion polymorphism in FADS2 influences apparent endogenous synthesis of arachidonic acid. Mol Biol Evol 33(7):1726–1739
Janssen CI, Kiliaan AJ (2014) Long-chain polyunsaturated fatty acids (LCPUFA) from genesis to senescence: the influence of LCPUFA on neural development, aging, and neurodegeneration. Prog Lipid Res 53:1–17
Larque E, Ruiz-Palacios M, Koletzko B (2013) Placental regulation of fetal nutrient supply. Curr Opin Clin Nutr Metab Care 16(3):292–297
Brenna JT, Varamini B, Jensen RG, Diersen-Schade DA, Boettcher JA, Arterburn LM (2007) Docosahexaenoic and arachidonic acid concentrations in human breast milk worldwide. Am J Clin Nutr 85(6):1457–1464
Luxwolda MF, Kuipers RS, Koops JH, Muller S, de Graaf D, Dijck-Brouwer DA, Muskiet FA (2014) Interrelationships between maternal DHA in erythrocytes, milk and adipose tissue. Is 1 wt% DHA the optimal human milk content? Data from four Tanzanian tribes differing in lifetime stable intakes of fish. Br J Nutr 111(5):854–866
Schmeits BL, Cook JA, Vander DJ, Magnussen MA, Bhatt SK (1999) Fatty acid composition of the milk lipids of women in Nepal. Nutr Res 9:1339–1348
Much D, Brunner S, Vollhardt C, Schmid D, Sedlmeier EM, Bruderl M, Heimberg E, Bartke N, Boehm G, Bader BL, Amann-Gassner U, Hauner H (2013) Breast milk fatty acid profile in relation to infant growth and body composition: results from the INFAT study. Pediatr Res 74(2):230–237
Martin MA, Lassek WD, Gaulin SJ, Evans RW, Woo JG, Geraghty SR, Davidson BS, Morrow AL, Kaplan HS, Gurven MD (2012) Fatty acid composition in the mature milk of Bolivian forager-horticulturalists: controlled comparisons with a US sample. Matern Child Nutr 8(3):404–418
Luxwolda MF, Kuipers RS, Sango WS, Kwesigabo G, Dijck-Brouwer DA, Muskiet FA (2012) A maternal erythrocyte DHA content of approximately 6 g% is the DHA status at which intrauterine DHA biomagnifications turns into bioattenuation and postnatal infant DHA equilibrium is reached. Eur J Nutr 51(6):665–675
Kuipers RS, Luxwolda MF, Sango WS, Kwesigabo G, Dijck-Brouwer DA, Muskiet FA (2011) Maternal DHA equilibrium during pregnancy and lactation is reached at an erythrocyte DHA content of 8 g/100 g fatty acids. J Nutr 141(3):418–427
Glew RH, Huang YS, Vander Jagt TA, Chuang LT, Bhatt SK, Magnussen MA, VanderJagt DJ (2001) Fatty acid composition of the milk lipids of Nepalese women: correlation between fatty acid composition of serum phospholipids and melting point. Prostaglandins Leukot Essent Fatty Acids 65(3):147–156
Lee PS, Wickramasinghe VP, Lamabadusuriya SP, Duncan AW, Wainscott G, Weeraman JD, Wijekoon AS, Wong KH (2013) Breast milk DHA levels in Sri Lankan mothers vary significantly in three locations that have different access to dietary fish. Ceylon Med J 58(2):51–55
Glew RH, VanderJagt DJ (2013) Does docosahexaenoic acid play a role in infant malnutrition in the children of Fulani nomads in Northern Nigeria? J Med Trop 15(2):69–75
Adu-Afarwuah, Lartey L, Brown KH, Zlotkin S, Briend A, Dewey KG (2007) A fat-based supplement containing essential fatty acids increased plasma {alpha}-linolenic acid and linear growth of Ghanaian infants. FASEB J 21(5):223–227
Arimond M, Zeilani M, Jungjohann S, Brown KH, Ashorn P, Allen LH, and Dewey KG (2015) Considerations in developing lipid-based nutrient supplements for prevention of undernutrition: experience from the International Lipid-Based Nutrient Supplements (iLiNS) Project. Matern Child Nutr. 11(Suppl 4):31–61
Barbarich BN, Willows ND, Wang L, Clandinin MT (2006) Polyunsaturated fatty acids and anthropometric indices of children in rural China. Eur J Clin Nutr 60(9):1100–1107
Bascunan KA, Valenzuela R, Chamorro R, Valencia A, Barrera C, Puigrredon C, Sandoval J, Valenzuela A (2014) Polyunsaturated fatty acid composition of maternal diet and erythrocyte phospholipid status in Chilean pregnant women. Nutrients 6(11):4918–4934
Raatz SK, Young LR, Picklo MJ Sr, Sauter ER, Qin W, Kurzer MS (2012) Total dietary fat and fatty acid content modifies plasma phospholipid fatty acids, desaturase activity indices, and urinary prostaglandin E in women. Nutr Res 32(1):1–7
Raatz SK, Bibus D, Thomas W, Kris-Etherton P (2001) Total fat intake modifies plasma fatty acid composition in humans. J Nutr 131(2):231–234
Markhus MW, Rasinger JD, Malde MK, Froyland L, Skotheim S, Braarud HC, Stormark KM, Graff IE (2015) Docosahexaenoic Acid Status in Pregnancy Determines the Maternal Docosahexaenoic Acid Status 3-, 6- and 12 Months Postpartum. Results from a Longitudinal Observational Study. PLoS One 10(9):e0136409
Warensjo E, Rosell M, Hellenius ML, Vessby B, De Faire U, Riserus U (2009) Associations between estimated fatty acid desaturase activities in serum lipids and adipose tissue in humans: links to obesity and insulin resistance. Lipids Health Dis 8:37
Matthan NR, Ooi EM, Van Horn L, Neuhouser ML, Woodman R, Lichtenstein AH (2014) Plasma phospholipid fatty acid biomarkers of dietary fat quality and endogenous metabolism predict coronary heart disease risk: a nested case-control study within the Women’s Health Initiative observational study. J Am Heart Assoc 3(4):e000764
Markhus MW, Skotheim S, Graff IE, Froyland L, Braarud HC, Stormark KM, Malde MK (2013) Low omega-3 index in pregnancy is a possible biological risk factor for postpartum depression. PLoS One 8(7):e67617
EFSA (2010) Scientific Opinion on Dietary Reference Values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. EFSA J 8(3)107
Burns CE, Dunn AM, Brady MA, Starr NB, Blosser CG, Garzon DL (2016) Pediatric Primary Care 6th Edition ed. Missouri: Elsevier. 162
Abul-Fadl MM, El-Badry N, Ammar MS (2011) Nutritional and Chemical Evaluation for Two Different Varieties of Mustard Seeds. World Appl Sci J 15(9):1225–1233
Dubois V, Breton S, Linder M, Fanni J, Parmentier M (2007) Fatty acid profiles of 80+ vegetable oils with regard to their nutritional potential. Eur J Lipid Sci Technol 109(7):710–732
EFSA (2013) Scientific Opinion on nutrient requirements and dietary intakes of infants and young children in the European Union. EFSA J 11(10):3408
European Union Directives (1976) Council Directive 76/621/EEC. Off J Eur Commun 76(621):35–37
Acknowledgements
We want to thank the children and their families for participating in this study. We are also grateful to the staff at Siddhi Memorial Hospital and the fieldworkers. Funding: Supported by Research Council of Norway (Project No. 172226), and a grant from the GCRieber Funds, South-Eastern Norway Regional Health Authority (Grant No. 2012090) and by the USAID Feed the Future Innovation Laboratory for Nutrition.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Henjum, S., Lie, Ø., Ulak, M. et al. Erythrocyte fatty acid composition of Nepal breast-fed infants. Eur J Nutr 57, 1003–1013 (2018). https://doi.org/10.1007/s00394-017-1384-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-017-1384-4