Abstract
Purpose
The study aimed to determine the effects of maternal low-protein (LP) diet on subcutaneous fat deposition of weaning piglets and the potential mechanism.
Methods
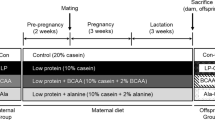
Sows were fed either a standard protein (SP, 15 and 18% crude protein) or a LP diet (50% protein levels of SP) throughout pregnancy and lactation. Subcutaneous fat and blood were sampled from male piglets at 28 days of age. Serum biochemical metabolites and hormone concentrations were detected with the enzymatic colorimetric methods. Serum-free amino acid (FAA) levels were measured by amino acid auto-analyzer. The mRNA and protein were measured by qRT-PCR and Western blot.
Results
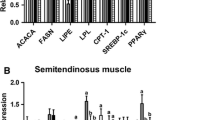
Body weight, back fat thickness, triglycerides concentrations in subcutaneous fat tissue, and serum, as well as FFA concentrations were significantly reduced in LP piglets when compared with SP piglets. Further studies showed that mRNA and protein expression of acetyl-CoA carboxylase and fatty acid synthetase, two key enzymes of de novo lipogenesis, were significantly reduced in LP piglets, while mRNA expression and the lipolytic enzymes activities of lipolysis genes, adipose triglyceride lipase and hormone-sensitive lipase, were significantly increased. Furthermore, expression of autophagy-related gene 7 and autophagy maker gene microtubule-associated protein 1A/1B-light chain 3 (LC 3) as well as the conversion of LC3I to LC3II were significantly elevated, along with the expression of activating transcription factor-4 and eukaryotic translation initiation factor-2a.
Conclusion
These results indicate that amino acid starvation-induced autophagy is involved in reduced subcutaneous fat deposition in maternal LP weaning piglets, demonstrating links between maternal protein restriction and offspring fat deposition.



Similar content being viewed by others
Abbreviations
- AAR:
-
Amino acid response
- ACC:
-
Acetyl coenzyme A carboxylase
- ATF4:
-
Activating transcription factor 4
- ATG7:
-
Autophagy-related gene 7
- ATGL:
-
Adipose triglyceride lipase
- C/EBP-β:
-
CCAAT–enhancer-binding proteins-β
- Eif2α:
-
Eukaryotic initiation factor 2α
- FAS:
-
Fatty acid synthetase
- FFA:
-
Free amino acid
- GR:
-
Glucocorticoid receptor
- HSL:
-
Hormone-sensitive lipase
- LC3:
-
Microtubule-associated protein 1A/1B-light chain 3
- LDs:
-
Lipid droplets
- LP:
-
Low-protein
- PERK:
-
Protein kinase R-like endoplasmic reticulum kinase
- p-eif2α:
-
Phosphorylated eukaryotic initiation factor 2α
- PPAR-γ:
-
Peroxisome proliferator-activated receptor-γ
- RT-PCR:
-
Quantitative real-time polymerase chain reaction
- SCD-1:
-
Stearoyl-coenzyme A desaturase 1
- SP:
-
Standard protein
- SPSS:
-
Statistical program for social sciences
- TG:
-
Triglycerides
- XBP-1:
-
X box-binding protein-1
References
Bruce KD, Hanson MA (2010) The developmental origins, mechanisms, and implications of metabolic syndrome[J]. J Nutr 140:648–652
Martinez MA, Puig JG, Mora M, Aragon R, O’Dogherty P, Anton JL, Sanchez-Villares T, Rubio JM, Rosado J, Torres R, Marcos J, Pallardo LF, Banegas JR (2008) Metabolic syndrome: prevalence, associated factors, and C-reactive protein: the MADRIC (MADrid RIesgo Cardiovascular) Study[J]. Metabolism 57:1232–1240
Reaven GM (1988) Role of insulin resistance in human disease[J]. Diabetes 37:1595–1607
Cezar de Oliveira J, Gomes RM, Miranda RA, Barella LF, Malta A, Martins IP, da Silva Franco CC, Pavanello A, Torrezan R, Marcal Natali MR, Lisboa PC, de Freitas Mathias PC, Gaspar de Moura E (2016) Protein-restriction during the last third of pregnancy malprograms the neuroendocrine axes to induce metabolic syndrome in adult male rat offspring[J]. Endocrinology 157:1799–1812
Zambrano E, Bautista CJ, Deas M, Martinez-Samayoa PM, Gonzalez-Zamorano M, Ledesma H, Morales J, Larrea F, Nathanielsz PW (2006) A low maternal protein diet during pregnancy and lactation has sex- and window of exposure-specific effects on offspring growth and food intake, glucose metabolism and serum leptin in the rat[J]. J Physiol 571:221–230
Rehfeldt C, Lang IS, Gors S, Hennig U, Kalbe C, Stabenow B, Brussow KP, Pfuhl R, Bellmann O, Nurnberg G, Otten W, Metges CC (2011) Limited and excess dietary protein during gestation affects growth and compositional traits in gilts and impairs offspring fetal growth[J]. J Anim Sci 89:329–341
Cheim LM, Oliveira EA, Arantes VC, Veloso RV, Reis MA, Gomes-da-Silva MH, Carneiro EM, Boschero AC, Latorraca MQ (2009) Effect of nutritional recovery with soybean flour diet on body composition, energy balance and serum leptin concentration in adult rats[J]. Nutr Metab 6:34
Liang J, Zhang XW, Zhao RQ, Steffen M, Yang XJ (2011) Effect of maternal protein restriction on lipid metabolism in Meishan piglets at weaning[J]. Livest Sci 136:157–163
Rehfeldt C, Lefaucheur L, Block J, Stabenow B, Pfuhl R, Otten W, Metges CC, Kalbe C (2012) Limited and excess protein intake of pregnant gilts differently affects body composition and cellularity of skeletal muscle and subcutaneous adipose tissue of newborn and weanling piglets[J]. Eur J Nutr 51:151–165
Ameer F, Scandiuzzi L, Hasnain S, Kalbacher H, Zaidi N (2014) De novo lipogenesis in health and disease[J]. Metabolism 63:895–902
Zimmermann R, Strauss JG, Haemmerle G, Schoiswohl G, Birner-Gruenberger R, Riederer M, Lass A, Neuberger G, Eisenhaber F, Hermetter A, Zechner R (2004) Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase[J]. Science 306:1383–1386
Singh R, Cuervo AM (2012) Lipophagy: connecting autophagy and lipid metabolism[J]. Int. J Cell Biol 2012:282041
Settembre C, Ballabio A (2014) Lysosome: regulator of lipid degradation pathways[J]. Trends Cell Biol 24:743–750
Singh R, Xiang Y, Wang Y, Baikati K, Cuervo AM, Luu YK, Tang Y, Pessin JE, Schwartz GJ, Czaja MJ (2009) Autophagy regulates adipose mass and differentiation in mice[J]. J Clin Invest 119:3329–3339
Baerga R, Zhang Y, Chen PH, Goldman S, Jin S (2009) Targeted deletion of autophagy-related 5 (atg5) impairs adipogenesis in a cellular model and in mice[J]. Autophagy 5:1118–1130
Nishida Y, Arakawa S, Fujitani K, Yamaguchi H, Mizuta T, Kanaseki T, Komatsu M, Otsu K, Tsujimoto Y, Shimizu S (2009) Discovery of Atg5/Atg7-independent alternative macroautophagy[J]. Nature 461:654–658
Hao S, Sharp JW, Ross-Inta CM, McDaniel BJ, Anthony TG, Wek RC, Cavener DR, McGrath BC, Rudell JB, Koehnle TJ, Gietzen DW (2005) Uncharged tRNA and sensing of amino acid deficiency in mammalian piriform cortex[J]. Science 307:1776–1778
Thiaville MM, Dudenhausen EE, Zhong C, Pan YX, Kilberg MS (2008) Deprivation of protein or amino acid induces C/EBPbeta synthesis and binding to amino acid response elements, but its action is not an absolute requirement for enhanced transcription[J]. Biochem J 410:473–484
Zhou D, Pan YX (2011) Gestational low protein diet selectively induces the amino acid response pathway target genes in the liver of offspring rats through transcription factor binding and histone modifications[J]. Biochim Biophys Acta 1809:549–556
Sahani MH, Itakura E, Mizushima N (2014) Expression of the autophagy substrate SQSTM1/p62 is restored during prolonged starvation depending on transcriptional upregulation and autophagy-derived amino acids[J]. Autophagy 10:431–441
Rzymski T, Milani M, Singleton DC, Harris AL (2009) Role of ATF4 in regulation of autophagy and resistance to drugs and hypoxia[J]. Cell Cycle 8:3838–3847
Rzymski T, Milani M, Pike L, Buffa F, Mellor HR, Winchester L, Pires I, Hammond E, Ragoussis I, Harris AL (2010) Regulation of autophagy by ATF4 in response to severe hypoxia[J]. Oncogene 29:4424–4435
Rouschop KM, van den Beucken T, Dubois L, Niessen H, Bussink J, Savelkouls K, Keulers T, Mujcic H, Landuyt W, Voncken JW, Lambin P, van der Kogel AJ, Koritzinsky M, Wouters BG (2010) The unfolded protein response protects human tumor cells during hypoxia through regulation of the autophagy genes MAP1LC3B and ATG5[J]. J Clin Invest 120:127–141
Wang H, Wilson GJ, Zhou D, Lezmi S, Chen X, Layman DK, Pan YX (2015) Induction of autophagy through the activating transcription factor 4 (ATF4)-dependent amino acid response pathway in maternal skeletal muscle may function as the molecular memory in response to gestational protein restriction to alert offspring to maternal nutrition[J]. Br J Nutr 114:519–532
Wu G, Knabe DA (1994) Free and protein-bound amino acids in sow’s colostrum and milk[J]. J Nutr 124:415–424
Zhang TT, Xu C, Zu LX, He JH, Pu SS, Guo XH, Xu GH (2008) The mechanisms of stimulated lipolysis by high concentration of glucose in primary rat adipocytes[J]. Beijing Da Xue Xue Bao 40:273–279
Bol VV, Delattre AI, Reusens B, Raes M, Remacle C (2009) Forced catch-up growth after fetal protein restriction alters the adipose tissue gene expression program leading to obesity in adult mice[J]. Am J Physiol Regul Integr Comp Physiol 297:R291–R299
Metges CC, Görs S, Lang IS, Hammon HM, Brüssow KP, Weitzel JM, Nürnberg G, Rehfeldt C, Otten W (2014) Low and high dietary protein:carbohydrate ratios during pregnancy affect materno-fetal glucose metabolism in pigs. J Nutr 144:155–163
Sarr O, Louveau I, Kalbe C, Metges CC, Rehfeldt C, Gondret F (2010) Prenatal exposure to maternal low or high protein diets induces modest changes in the adipose tissue proteome of newborn piglets. J Anim Sci 88:1626–1641
Rehfeldt C, Stabenow B, Pfuhl R, Block J, Nürnberg G, Otten W, Metges CC, Kalbe C (2012) Effects of limited and excess protein intakes of pregnant gilts on carcass quality and cellular properties of skeletal muscle and subcutaneous adipose tissue in fattening pigs. J Anim Sci 90:184–196
Erhuma A, Salter AM, Sculley DV, Langley-Evans SC, Bennett AJ (2007) Prenatal exposure to a low-protein diet programs disordered regulation of lipid metabolism in the aging rat[J]. Am J Physiol Endocrinol Metab 292:E1702–E1714
Zhang T, Guan H, Arany E, Hill DJ, Yang K (2007) Maternal protein restriction permanently programs adipocyte growth and development in adult male rat offspring[J]. J Cell Biochem 101:381–388
McMillen IC, Adam CL, Muhlhausler BS (2005) Early origins of obesity: programming the appetite regulatory system[J]. J Physiol 565:9–17
Holzhauer S, Hokken Koelega AC, Ridder M, Hofman A, Moll HA, Steegers EA, Witteman JC, Jaddoe VW (2009) Effect of birth weight and postnatal weight gain on body composition in early infancy: The Generation R Study[J]. Early Hum Dev 85:285–290
Jia Y, Gao G, Song H, Cai D, Yang X, Zhao R (2016) Low-protein diet fed to crossbred sows during pregnancy and lactation enhances myostatin gene expression through epigenetic regulation in skeletal muscle of weaning piglets. Eur J Nutr 55:1307–1314
Dwyer CM, Stickland NC, Fletcher JM (1994) The influence of maternal nutrition on muscle fiber number development in the porcine fetus and on subsequent postnatal growth[J]. J Anim Sci 72:911–917
Woods LL, Ingelfinger JR, Nyengaard JR, Rasch R (2001) Maternal protein restriction suppresses the newborn renin-angiotensin system and programs adult hypertension in rats[J]. Pediatr Res 49:460–467
Yuasa K, Kondo T, Nagai H, Mino M, Takeshita A, Okada T (2016) Maternal protein restriction that does not have an influence on the birthweight of the offspring induces morphological changes in kidneys reminiscent of phenotypes exhibited by intrauterine growth retardation rats[J]. Congenit Anom 56:79–85
Lucas A, Baker BA, Desai M, Hales CN (1996) Nutrition in pregnant or lactating rats programs lipid metabolism in the offspring[J]. Br J Nutr 76:605–612
Pan S, Zheng Y, Zhao R, Yang X (2013) MicroRNA-130b and microRNA-374b mediate the effect of maternal dietary protein on offspring lipid metabolism in Meishan pigs[J]. Br J Nutr 109:1731–1738
Li Y, Zheng X, Yang G (2008) Effects of leptin on porcine primary adiocytes lipolysis and mRNA expression of key lipolytic enzymes[J]. Sheng Wu Gong Cheng Xue Bao 24:1613–1619
Kwon H, Ford SP, Bazer FW, Spencer TE, Nathanielsz PW, Nijland MJ, Hess BW, Wu G (2004) Maternal nutrient restriction reduces concentrations of amino acids and polyamines in ovine maternal and fetal plasma and fetal fluids[J]. Biol Reprod 71:901–908
Wu G, Pond WG, Ott T, Bazer FW (1998) Maternal dietary protein deficiency decreases amino acid concentrations in fetal plasma and allantoic fluid of pigs. J Nutr 128:894–902
Kilberg MS, Shan J, Su N (2009) ATF4-dependent transcription mediates signaling of amino acid limitation[J]. Trends Endocrinol Metab 20:436–443
Kilberg MS, Pan YX, Chen H, Leung-Pineda V (2005) Nutritional control of gene expression: how mammalian cells respond to amino acid limitation[J]. Annu Rev Nutr 25:59–85
Strakovsky RS, Zhou D, Pan YX (2010) A low-protein diet during gestation in rats activates the placental mammalian amino acid response pathway and programs the growth capacity of offspring[J]. J Nutr 140:2116–2120
Marchianti AC, Arimura E, Ushikai M, Horiuchi M (2014) Voluntary exercise under a food restriction condition decreases blood branched-chain amino acid levels, in addition to improvement of glucose and lipid metabolism, in db mice, animal model of type 2 diabetes[J]. Environ Health Prev Med 19:339–347
Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, Tanaka K, Cuervo AM, Czaja MJ (2009) Autophagy regulates lipid metabolism[J]. Nature 458:1131–1135
Ward C, Martinez-Lopez N, Otten EG, Carroll B, Maetzel D, Singh R, Sarkar S, Korolchuk VI (2016) Autophagy, lipophagy and lysosomal lipid storage disorders[J]. Biochim Biophys Acta 1864:269–284
Acknowledgements
We thank Shanghai Farm of Bright Food (Group) Co., Ltd., for providing the experimental site and Rongkui Zhang for care of animals. In addition, we are grateful to members of our laboratories for critically reviewing the manuscript and for helpful discussion. This work was supported by the National Basic Research Program of China (2012CB124703), the Fundamental Research Funds for the Central Universities (KYZ200913), the Major National Science & Technology Program (2009ZX08009-138B), the Special Fund for Agro-scientific Research in the Public Interest (201003011), and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflicts of interest, financial or otherwise, are declared by the authors.
About this article
Cite this article
Pan, S., Jia, Y., Yang, X. et al. Amino acid starvation-induced autophagy is involved in reduced subcutaneous fat deposition in weaning piglets derived from sows fed low-protein diet during gestation and lactation. Eur J Nutr 57, 991–1001 (2018). https://doi.org/10.1007/s00394-017-1383-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-017-1383-5