Abstract
Purpose
To investigate the mechanistic effects of combined exposure to caffeine and catechins on lipid metabolism in mice.
Methods
Seventy mice were randomly assigned to seven groups and fed diets containing varying doses of caffeine and catechins for 24 weeks. Body weight gain, intraperitoneal adipose tissue (IPAT) weight, serum biochemical parameters, and enzymatic activities, mRNA and protein expression levels of lipid metabolism-related enzymes in the liver and IPAT were analyzed.
Results
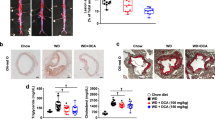
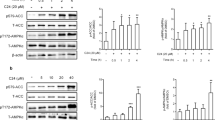
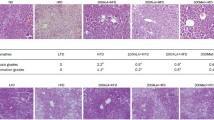
Following administration of caffeine and catechins, body weight gain, IPAT weight, serum and liver concentrations of total cholesterol and triglyceride were markedly reduced. Lipase activities, including that of AMP-activated protein kinase (AMPK), acyl-CoA oxidase, carnitine acyltransferase, adipose triglyceride lipase, and hormone-sensitive lipase, were significantly upregulated; however, fatty acid synthase (FAS) activity in the liver was suppressed. Combined exposure to caffeine and catechins significantly upregulated mRNA and protein expression levels of lipases while downregulating FAS mRNA expression and protein expression of peroxisome proliferator-activated receptor γ2.
Conclusions
The combination of caffeine and catechins regulated the enzymatic activities, mRNA, and protein expression levels of lipid metabolism-related enzymes, resulting in suppression of body weight gain and IPAT weight in mice, potentially through activation of the AMPK signaling pathway. This study indicates that chronic intake of both caffeine and catechins can synergistically contribute to prevention of obesity and lifestyle-related diseases.


Similar content being viewed by others
Abbreviations
- AMPK:
-
AMP-activated protein kinase
- ATGL:
-
Adipose triglyceride lipase
- HSL:
-
Hormone-sensitive lipase
- ACC:
-
Acetyl-CoA carboxylase
- IPAT:
-
Intraperitoneal adipose tissue
- TC:
-
Total cholesterol
- TG:
-
Triglyceride
- CAT:
-
Carnitine acyltransferase
- ACO:
-
Acyl-CoA oxidase
- FAS:
-
Fatty acid synthase
- PPARγ2:
-
Peroxisome proliferator-activated receptor γ2
- p-HSL (Ser660):
-
Phosphorylation of hormone-sensitive lipase Ser660
- SREBP-1c:
-
Sterol-responsive element-binding protein-1c
- WAT:
-
White adipose tissue
References
Gaidhu MP, Ceddia RB (2011) The role of adenosine monophosphate kinase in remodeling white adipose tissue metabolism. Exerc Sport Sci Rev 39(2):102–108
Lee CE, Hur HJ, Hwang JT, Sung MJ, Yang HJ, Kim HJ, Park JH, Kwon DY, Kim MS (2012) Long-term consumption of platycodi radix ameliorates obesity and insulin resistance via the activation of AMPK pathways. Evid Based Complement Alternat Med 2012:759143. doi:10.1155/2012/759143
Collins QF, Liu HY, Pi J, Liu Z, Quon MJ, Cao W (2007) Epigallocatechin-3-gallate (EGCG), a green tea polyphenol, suppresses hepatic gluconeogenesis through 5′-AMP-activated protein kinase. J Biol Chem 282(41):30143–30149
Sun Y, Mukai Y, Tanaka M, Saito T, Sato S, Kurasaki M (2013) Green tea extract increases mRNA expression of enzymes which influence epigenetic marks in newborn female offspring from undernourished pregnant mother. PLoS One 8(8):e74559
Gramza A, Korczak J, Amarowicz R (2005) Tea polyphenols-their antioxidant properties and biological activity—a review. Pol J Food Nutr Sci 14(3):219–235
Ajmo JM, Liang X, Rogers CQ, Pennock B, You M (2008) Resveratrol alleviates alcoholic fatty liver in mice. Am J Physiol Gastrointest Liver Physiol 295(4):G833–G842
Eid HM, Martineau LC, Saleem A, Muhammad A, Vallerand D, Benhaddou-Andaloussi A, Nistor L, Afshar A, Arnason JT, Haddad PS (2010) Stimulation of AMP-activated protein kinase and enhancement of basal glucose uptake in muscle cells by quercetin and quercetin glycosides, active principles of the antidiabetic medicinal plant vaccinium vitis-idaea. Mol Nutr Food Res 54(7):991–1003
Dolinsky VW, Rueda-Clausen CF, Morton JS, Davidge ST, Dyck JRB (2011) Continued postnatal administration of resveratrol prevents diet-induced metabolic syndrome in rat offspring born growth restricted. Diabetes 60(9):2274–2284
Gaidhu MP, Fediuc S, Anthony NM, So M, Mirpourian M, Perry RL, Ceddia RB (2009) Prolonged AICAR-induced AMP-kinase activation promotes energy dissipation in white adipocytes: novel mechanisms integrating HSL and ATGL. J Lipid Res 50(4):704–715
Whitehead JP, Richards AA, Hickman IJ, Macdonald GA, Prins JB (2006) Adiponectin-akey adipokine in the metabolic syndrome. Diabetes Obes Metab 8(3):264–280
Zhang BB, Zhou G, Li C (2009) AMPK: an emerging drug target for diabetes and the metabolic syndrome. Cell Metab 9(5):407–416
Viollet B, Guigas B, Leclerc J, Hébrard S, Lantier L, Mounier R, Andreelli F, Foretz M (2009) AMP-activated protein kinase in the regulation of hepatic energy metabolism: from physiology to therapeutic perspectives. Acta Physiol 196(1):81–98
Zang M, Xu S, Maitland-Toolan KA, Zuccollo A, Hou X, Jiang B, Wierzbicki M, Verbeuren TJ, Cohen RA (2006) Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deicient mice. Diabetes 55(8):2180–2191
Gao Y, Zhou Y, Xu A, Wu D (2008) Effects of an AMP-activated protein kinase inhibitor, compound C, on adipogenic differentiation of 3T3–L1 cells. Biol Pharm Bull 31(9):1716–1722
Habinowski SA, Witters LA (2001) The effects of AICAR on adipocyte differentiation of 3T3-L1 cells. Biochem Biophys Res Commun 286(5):852–856
Lee H, Kang R, Bae S, Yoon Y (2011) AICAR, an activator of AMPK, inhibits adipogenesis via the WNT/b-catenin pathway in 3T3-L1 adipocytes. Int J Neural Mol Med 28(1):65–71
Zheng G, Sayama K, Okubo T, Juneja LR, Oguni I (2004) Anti-obesity effects of three major components of green tea, catechins, caffeine and theanine, in mice. Vivo 18(1):55–62
Folch J, Lees M, Sloane-Stanley GH (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226(1):497–509
Zheng G, Qiu Y, Zhang Q, Li D (2014) Chlorogenic acid and caffeine in combination inhibit fat accumulation by regulating hepatic lipid metabolism-related enzymes in mice. Br J Nutr 112(06):1034–1040
Markwell MA, McGroarty EJ, Bieber LL, Tolbert NE (1973) The subcellular distribution of carnitine acyltransferases in mammalian liver and kidney. J Boil Chem 248(10):3426–3432
Osumi T, Hashimoto T (1978) Acyl-CoA oxidase of rat liver: a new enzyme for fatty acid oxidation. Biochem Biophys Res Commun 83(2):479–485
Kelley DS, Nelson GJ, Hunt JE (1986) Effect of prior nutritional status on the activity of lipogenic enzymes in primary monolayer cultures of rat hepatocytes. Biochem J 235:87–90
Zheng G, Lin L, Zhong S, Zhang Q, Li D (2015) Effects of puerarin on lipid accumulation and metabolism in high-fat diet-fed mice. PLoS One 10(3):e0122925
Sanders MJ, Grondin PO, Hegarty BD, Snowden MA, Carling D (2007) Investigating the mechanism for AMP activation of the AMP-activated protein kinase cascade. Biochem J 403:139–148
Foretz M, Ancellin N, Andreelli F, Saintillan Y, Grondin P, Kahn A, Thorens B, Vaulont S, Viollet B (2005) Short-term overexpression of a constitutively active form of AMP-activated protein kinase in the liver leads to mild hypoglycemia and fatty liver. Diabetes 54(5):1331–1339
Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N (2001) Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 108(8):1167–1174
Kawaguchi T, Osatomi K, Yamashita H, Kabashima T, Uyeda K (2002) Mechanism for fatty acid “sparing” effect on glucose-induced transcription regulation of carbohydrate-responsive element-binding protein by AMP-activated protein kinase. J Biol Chem 277(6):3829–3835
Deng X, Dong Q, Bridges D, Raghow R, Park EA, Elam MB (1851) Docosahexaenoic acid inhibits proteolytic processing of sterol regulatory element-binding protein-1c (SREBP-1c) via activation of AMP-activated kinase. Biochim Biophys Acta Mol Cell Biol Lipids 12:1521–1529
Tang QQ, Gronborg M, Huang H, Kim JW, Otto TC, Pandey A, Lane MD (2005) Sequential phosphorylation of CCAAT enhancer-binding protein beta by MAPK and glycogen synthase kinase 3β is required for adipogenesis. Proc Natl Acad Sci USA 102(28):9766–9771
Sozio MS, Lu C, Zeng Y, Liangpunsakul S, Crabb DW (2011) Activated AMPK inhibits PPAR-α and PPAR-γ transcriptional activity in hepatoma cells. Am J Physiol Gastrointest Liver Physiol 301(4):G739–G747
Murase T, Nagasawa A, Suzuki J, Hase T, Tokimitsu I (2002) Beneficial effects of tea catechins on diet-induced obesity: stimulation of lipid catabolism in the liver. Int J Obes Relat Metab Disord 26(11):1459–1464
Reid BN, Ables GP, Otlivanchik OA, Schoiswohl G, Zechner R, Blaner WS, Goldberg IJ, Schwabe RF, Chua SC, Huang LS (2008) Hepatic overexpression of hormone-sensitive lipase and adipose triglyceride lipase promotes fatty acid oxidation, stimulates direct release of free fatty acids, and ameliorates steatosis. J Biol Chem 283(19):13087–13099
Zhu W, Chen S, Li Z, Zhao X, Li W, Sun Y, Zhang Z, Ling W, Feng X (2014) Effects and mechanisms of resveratrol on the amelioration of oxidative stress and hepatic steatosis in KKAy mice. Nutr Metab (Lond) 11(1):35–45
Haemmerle G, Lass A, Zimmermann R, Gorkiewicz G, Meyer C, Rozman J, Heldmaier G, Maier R, Theussl C, Eder S (2006) Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science 312(5774):734–737
Sekar R, Chow BK (2014) Lipolytic actions of secretin in mouse adipocytes. J Lipid Res 55(2):190–200
Holm C (2003) Molecular mechanisms regulating hormone-sensitive lipase and lipolysis. Biochem Soc Trans 31(6):1120–1124
Kraemer FB, Shen W (2002) Hormone-sensitive lipase control of intracellular tri-(di-)-acylglycerol and cholesteryl ester hydrolysis. J Lipid Res 43(10):1585–1594
Hurley RL, Barré LK, Wood SD, Anderson KA, Kemp BE, Means AR, Witters LA (2006) Regulation of AMP-activated protein kinase by multisite phosphorylation in response to agents that elevate cellular cAMP. J Biol Chem 281(48):36662–36672
Stark R, Ashley SE, Andrews ZB (2013) AMPK and the neuroendocrine regulation of appetite and energy expenditure. Mol Cell Endocrinol 366(2):215–223
Janečková R (2001) The role of leptin in human physiology and pathophysiology. Physiol Res 50:443–459
Chen NG, Swick AG, Romsos DR (1997) Leptin constrains acetylcholine-induced insulin secretion from pancreatic islets of ob/ob mice. J Clin Invest 100(5):1174–1179
Acknowledgments
This work was kindly supported by the National Natural Science Foundation of China (No. 31160320) and the Jiangxi Province Natural Science Foundation of China (No. 20142BAB204003).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethics statement
This study was carried out in strict accordance with the recommendations from the Guide for the Care and Use of Laboratory Animals of the Chinese Association for Laboratory Animal Science. All animal care and protocols were approved by the Animal Care and Use Committee of the Jiangxi Agricultural University. All killings were performed under sodium pentobarbital anesthesia, and efforts were taken to minimize animal suffering.
Additional information
Yan Zhao and Licong Yang have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhao, Y., Yang, L., Huang, Z. et al. Synergistic effects of caffeine and catechins on lipid metabolism in chronically fed mice via the AMP-activated protein kinase signaling pathway. Eur J Nutr 56, 2309–2318 (2017). https://doi.org/10.1007/s00394-016-1271-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-016-1271-4