Abstract
Purpose
Functional foods can prevent/reduce the risks related to obesity. Lentinula edodes is a highly nutritious mushroom rich in protein, vitamins and minerals. Some studies have demonstrated the hypocholesterolemic effects from L. edodes in high doses, which does not represent the consumption in humans. We evaluated ingestion of a realistic dose of L. edodes associated with a high-fat diet (HFD) on hematologic, biochemical and oxidative stress parameters.
Methods
Eighteen male Wistar rats were divided into three groups: control (normal diet); HFD; and HFD + L. edodes (100 mg/kg/day). After 30 days, blood was collected. Biochemical and hematologic parameters were analyzed, as well as oxidative stress biomarkers.
Results
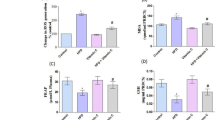
The HFD increased levels of total cholesterol and triglycerides. Lentinula edodes reduced these parameters significantly to concentrations found in the control group. The HFD increased levels of alanine transaminase and aspartate transaminase (markers of liver damage). Lentinula edodes returned the levels of these enzymes to normal levels and normalized serum levels of urea (which were also increased owing to consumption of the HFD). Lentinula edodes reduced levels of urea and glucose. Lipid peroxidation was increased in rats receiving the HFD, and L. edodes reduced malondialdehyde levels, thereby preventing oxidation of fatty acids.
Conclusions
Lentinula edodes was shown to have hypolipidemic, hypoglycemic, hepatoprotective and renoprotective features in doses that are suitable for humans.




Similar content being viewed by others
References
Landsberg L, Aronne LJ, Beilin LJ et al (2013) Obesity-related hypertension: pathogenesis, cardiovascular risk, and treatment–a position paper of the The Obesity Society and The American Society of Hypertension. Obesity 21(1):8–24. doi:10.1002/oby.20181
Carvalheira JBC, Qiu Y, Chawla A (2013) Blood spotlight on leukocytes and obesity. Am Soc Hematol 122:3263–3267. doi:10.1182/blood-201304-459446
WHO (2013) World Health Organization. Fact sheet no 311. Disponível em. http://www.who.int/mediacentre/factsheets/fs311/en/. Acesso em 29 de março de 2014
Hutcheson R, Rocic P (2012) The metabolic syndrome, oxidative stress, environment, and cardiovascular disease: the great exploration. Exp J Diabetes Res. doi:10.1155/2012/271028
Roupas P, Keogh J, Noakes M et al (2012) The role of edible mushrooms in health: evaluation of the evidence. J Funct Foods 4:687–709. doi:10.1016/j.jff.2012.05.003
Israilides C, Kletsas D, Arapoglou D et al (2008) In vitro cytostatic and immunomodulatory properties of the medicinal mushroom Lentinula edodes. Phytomedicine 15:512–519. doi:10.1016/j.phymed.2007.11.029
Wani BA, Bodha RH, Wani AH (2010) Nutritional and medicinal importance of mushrooms. JMPR 4:2598–2604. doi:10.5897/JMPR09.565
Regula J, Siwulski M (2007) Dried shiitake (Lentinulla edodes) and oyster (pleurotus ostreatus) mushrooms as a good source of nutrient. Acta Sci Pol Technol Aliment 6:135–142
Nile SH, Park W (2014) Total, soluble, and insoluble dietary fiber contents of wild growing edible mushrooms. Czech J Food Sci 32:302–307
Rop O, Mlcek J, Jurikova T (2009) Beta-glucans in higher fungi and their health effects nure. Nutr Rev 67:624–631. doi:10.1111/j.1753-4887.2009.00230
Giavasis L (2014) Bioactive fungal polysaccharides as potential functional ingredients in food and nutraceuticals. Curr Opin Chem Biol 26:162–173. doi:10.1016/j.copbio.2014.01.010
Ferreira ICFR, Barros L, Abreu RMV (2009) Antioxidants in wild mushrooms. Curr Med Chem 16:1543–1560
Hazama S, Watanabe S, Ohashi M et al (2009) Efficacy of orally administered superfine dispersed lentinan (β-1,3-glucan) for the treatment of advanced colorectal cancer. Anticancer Res 29:2611–2618
Attitalla IH (2011) Lentinus sp. RJ-2 mushroom is important source of natural antioxidative polysaccharides. PJBS 14:1070–1071. doi:10.3923/pjbs.2011.1070.1071
Kitzberger CSG, Smânia A Jr, Pedrosa RC et al (2007) Antioxidant and antimicrobial activities of shiitake (Lentinula edodes) extracts obtained by organic solvents and supercritical fluids. J Food Eng 80:631–638. doi:10.1016/j.jfoodeng.2006.06.013
Rincão VP, Yamamoto KA, Ricardo NMPS et al (2012) Polysaccharide and extracts from Lentinula edodes: structural features and antiviral activity. Virology 9:37. doi:10.1186/1743-422X-9-37
Guillamón E, García-Lafuente A, Lozano M et al (2010) Edible mushrooms: role in the prevention of cardiovascular diseases. Fitoterapia 81:715–723. doi:10.1016/j.fitote.2010.06.005
Alam N, Yoon KN, Lee TS et al (2011) Hypolipidemic activities of dietary Pleurotus ostreatus in hypercholesterolemic rats. Mycobiology 39:45–51. doi:10.4489/MYCO.2011.39.1.045
Zhu M, Nie P, Liang Y, Wang B (2013) Optimizing conditions of polysaccharide extraction from shiitake mushroom using response surface methodology and its regulating lipid metabolism. Carbohydr Polym 95:644–648. doi:10.1016/j.carbpol.2013.03.035
Mircea C, Bild V, Zavastin D (2013) The protective effect of mushrooms in experimentally induced diabetes in Mice. Farmacia 61:268–275
Fortes RC, Recôva VL, Melo AL et al (2008) Effects of dietary supplementation with medicinal fungus in fasting glycemia levels of patients with colorectal cancer: a randomized, double-blind, placebo-controlled clinical study. Nutr Hosp 23:591–598
Araujo GS, Matos LJBL, Fernandes JO et al (2013) Extraction of lipids from microalgae by ultrasound application: prospection of the optimal extraction method. Ultrason Sonochem 20:95–98. doi:10.1016/j.ultsonch.2012.07.027
Zhou N, Li W, Wu Z et al (2015) Sequential extractions: a new way for protein quantification data from peanut allergens. Anal Biochem 484:31–36. doi:10.1016/j.ab.2015.05.013
Pinela J, Barros L, Carvalho AM et al (2012) Nutritional composition and antioxidant activity of four tomato (Lycopersicon esculentum L.) farmer’ varieties in Northeastern Portugal homegardens. Food Chem Toxicol 50:829–834. doi:10.1016/j.fct.2011.11.045
Mccleary BV, Devries JW, Plymouth AN et al (2010) Determination of total dietary fiber (CODEX Definition) by enzymatic-gravimetric method and liquid chromatography: collaborative study. J AOAC 93:221–233
Grotto D, Gerenutti M, Souza VCO et al (2015) Deficiency of macro-and micronutrients induced by Lentinula edodes. Toxicol Rep 2:401–404. doi:10.1016/j.fct.2011.11.045
Fraulob JC et al (2010) A mouse model of metabolic syndrome: insulin resistance, fatty liver and non-alcoholic fatty pancreas disease (NAFPD) in C57BL/6 mice fed a high fat diet. J Clin Biochem Nutr 46:212–223. doi:10.3164/jcbn.09-83
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77. doi:10.1016/0003-9861(59)90090-6
Paglia DE, Valentine WN (1967) Study on the quantitative and qualitative caracte- rization of erythrocyte glutathione peroxide. J Lab Clin Med 70:158–169
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126. doi:10.1016/S0076-6879(84)05016-3
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by Thiobarbituric Acid reaction. Analyt Biochem 95:351–358
Mattila P, Vaananen PS, Konko K et al (2002) Basic composition and amino acid contents of mushrooms cultivated in Finland. J Agric Food Chem 50:6419–6422
Reis FS, Barros L, Martins A et al (2012) Chemical composition and nutritional value of the most widely appreciated cultivated mushrooms: an inter-species comparative study. Food Chem Toxicol 50:191–197. doi:10.1016/j.fct.2011.10.056
Gaitán-Hernández R, Esqueda M, Gutiérrez A et al (2006) Bioconversion of agrowastes by Lentinula edodes: the high potential of viticulture residues. Appl Microbiol Biot 71:432–439. doi:10.1007/s00253-005-0241-1
Brauer D, Kimmons TE, Phillips M et al (2011) Starch concentrations in log-grown shiitake mushrooms (Lentinula edodes (Berk.) Pegler). TOMYCJ 5:1–7. doi:10.2174/1874437001105010001
Bak WC, Park JH, Park YA et al (2014) Determination of glucan contents in the fruiting bodies and mycelia of Lentinula edodes cultivars. Mycrobiology 42:301–304. doi:10.5941/MYCO.2014.42.3.301
Brauer D, Kimmons T, Phillips M (2007) Comparison of two methods for the quantitation of 13-glucans from shiitake mushrooms. J Herbs Spices Med Plants 13:15–26. doi:10.1300/J044vl3n03_02
Manzi P, Aguzzi A, Pizzoferrato L (2001) Nutritional value of mushrooms widely consumed in Italy. Food Chem 73:321–325. doi:10.1016/S0308-8146(00)00304-6
Handayani D, Chen J, Meyer BJ et al (2011) Dietary shiitake mushroom (Lentinus edodes) prevents fat deposition and lowers triglyceride in rats fed a high-fat diet. J Obes 2011:1–8. doi:10.1155/2011/258051
Ellinger VCM, Carlini LT, Moreira RO et al (2006) Relation between insulin resistance and hematological parameters in a brazilian sample. Arq Bras Endocrinol Metabol 50:114–117. doi:10.1590/S0004-27302006000100016
Barazzoni R, Cappellari GG, Semolic A et al (2014) The association between hematological parameters and insulin resistance Is modified by body mass index results from the North-East Italy MoMa population study. PLoS ONE 9:101590. doi:10.1371/journal.pone.0101590
Harada T, Miura N, Adachi Y et al (2002) Effect of SCG, 1,3-b-D-Glucan from sparassis crispa on the hematopoietic response in cyclophosphamide induced leukopenic mice. Biol Pharm Bull 25:931–939. doi:10.1248/bpb.25.931
Pini M, Gove ME, Sennello JA et al (2008) Role and regulation of adipokines during zymosan-induced peritoneal inflammation en mice. Endocrinology 149:4080–4085
Hernandéz GN, Dabin C, del Gayol C et al (2002) Haemorheological variables in a rat model of hypertriglyceridaemic obesity and diabetes. Vet Res Commun 26:625–635
Tai CJ, Chen CH, Chen HH et al (2010) Differential effect of high dietary fat intakes on haemorheological parameters in rat. Br J Nutr 103:977–983. doi:10.1017/S0007114509992704
Schindhelm RK, Diamant M, Dekker JM et al (2006) Alanine aminotransferase as a marker of non-alcoholic fatty liver disease in relation to type 2 diabetes mellitus and cardiovascular disease. Diabetes Metab Res Ver 22:437–443. doi:10.1002/dmrr.666
Akamatsu S, Watanabe A, Tamesada M et al (2004) Biol hepatoprotective effect of extracts from Lentinus edodes mycelia on dimethylnitrosamine-induced liver injury. Pharm Bull 27:1957–1960. doi:10.1248/bpb.27.1957
Wang D, Wei Y, Pagliassotti MJ (2006) Saturated fatty acids promote endoplasmic reticulum stress and liver injury in rats with hepatic steatosis. Endocrinology. doi:10.1210/en.2005-0570
Chung WS, Huawang J, Bose S et al (2015) Hepatoprotective effect of Lentinus edodes mycelia fermented formulation against alcoholic liver injury in rats. J Food Biochem. doi:10.1111/jfbc.12124
Vaidya VS, Ramirez V, Ichimura T et al (2006) Urinary kidney injury molecule-1: a sensitive quantitative biomarker for early detection of kidney tubular injury. Am J Physiol Renal Physiol 290:517–529. doi:10.1152/ajprenal.00291.2005
Cha JY, Jun BS, Lee CH et al (2005) Hypoglicemic and antioxidative effects of fermented Chaga Mushroom (inonotus obliquus) on streptozotocin-induced diabetic rats. J Life Sci 15:809–818. doi:10.5352/JLS.2005.15.5.809
Sheena N, Ajith TA, Janardhanan KK (2003) Prevention of nephrotoxicity induced by the anticancer drug cisplatin, using Ganoderma lucidum, a medicinal mushroom occurring in South India. Curr Sci 85:478–482
Fukushima M, Ohashi T, Fujiwara Y et al (2001) Cholesterol-lowering effects of maitake (Grifola frondosa) fiber, shiitake (Lentinus edodes) fiber, and enokitake (Flammulina velutipes) fiber in rats. Exp Biol Med 226:758–765
Yoon KN, Alam N, Lee JS et al (2011) Antihyperlipidemic effect of dietary Lentinus edodes on plasma, feces and hepatic tissues in hypercholesterolemic rats. Mycobiology 39:96–102. doi:10.4489/MYCO.2011.39.2.096
Yang H, Hwang I, Kim S et al (2013) Lentinus edodes promotes fat removal in hypercholesterolemic mice. Exp Ther Med 6:1409–1413. doi:10.3892/etm.2013.1333
Novak M, Vetvicka V (2008) Beta-Glucans, history, and the present: immunomodulatory aspects and mechanisms of action. J Immunotoxicol 5:47–57. doi:10.1080/15476910802019045
Jayakumar T, Ramesh E, Geraldine P (2006) Antioxidant activity of the oyster mushroom, Pleurotus ostreatus, on CCl4-induced liver injury in rats. Food Chem Toxicol 44:1989–1996
Kanagasabapathy G, Malek NA, Mahmood AA et al. (2013) Beta-glucan-rich extract from Pleurotus sajor-caju (Fr.) singer prevents obesity and oxidative stress in C57BL/6J mice fed on a high-fat diet. Hindawi 1–10, ID 185259. doi:10.1155/2013/185259
Acharya K, Chatterjee S, Biswas G et al (2012) Hepatoprotective effect of a wild edible mushroom on carbon tetrachloride-induced hepatotoxicity in mice. Int J Pharm Pharm Sci 4:285–288
Acknowledgments
The authors are grateful for financial support from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP-2013/05765-5) and Financiadora de Estudos e Projetos (FINEP-01.10.0659.00) and the Programa de Suporte à Pós-Graduação de Instituições de Ensino Particulares (PROSUP/CAPES). We also thank Thaisa Borim Pickler for technical support.
Funding sources
Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP-2013/05765-5) and Financiadora de Estudos e Projetos (FINEP-01.10.0659.00).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Spim, S.R.V., de Oliveira, B.G.C.C., Leite, F.G. et al. Effects of Lentinula edodes consumption on biochemical, hematologic and oxidative stress parameters in rats receiving high-fat diet. Eur J Nutr 56, 2255–2264 (2017). https://doi.org/10.1007/s00394-016-1266-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-016-1266-1