Abstract
Objectives
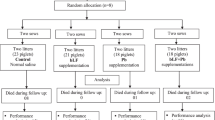
The aim of this study was to investigate the effects of intrauterine growth retardation (IUGR) and Bacillus subtilis PB6 supplementation in formula milk (FORM) on growth performance, intestinal development and immune function of neonates using a porcine model.
Methods
Fourteen pairs of normal birth weight and IUGR piglets (7 days old) were randomly assigned to receive FORM or FORM supplemented with B. subtilis PB6 (FORM-BsPB6) for a period of 21 days. Blood samples, intestinal tissues and digesta were collected at necropsy and analysed for morphology, digestive enzyme activities, immune cell abundance, expression of genes associated with innate immunity and barrier function and microbial populations.
Results
Regardless of diet, IUGR significantly decreased average daily dry matter intake and average daily weight gain (P < 0.05). Moreover, IUGR significantly decreased plasma concentrations of immunoglobulin A, interleukin 1β, count and percentage of blood lymphocytes (P < 0.05). Meanwhile, IUGR markedly decreased villous height and maltase activity, as well as mRNA abundance of Toll-like receptor 9 and Toll-interacting protein in the ileum (P < 0.05). Regardless of body weight, FORM-BsPB6 markedly decreased the feed conversion ratio (P < 0.05), due to better intestinal development, as indicated by increased villous height (P < 0.05), activities of maltase and sucrase in the intestine (P < 0.10). Moreover, both mRNA and protein abundances of zonula occludens-1 and claudin-1 in the ileum as well as the copy number of Bacillus in colonic digesta were increased (P < 0.05) in piglets fed FORM-BsPB6 relative to FORM.
Conclusion
The results of this study indicate that IUGR delayed growth, intestinal development and immune function of piglets, while FORM-BsPB6 improved digestive capability and intestinal barrier function.

Similar content being viewed by others
References
Wu G, Bazer FW, Wallace JM, Spencer TE (2006) Board-invited review: intrauterine growth retardation: implications for the animal sciences. J Anim Sci 84(9):2316–2337. doi:10.2527/jas.2006-156
Pallotto EK, Kilbride HW (2006) Perinatal outcome and later implications of intrauterine growth restriction. Clin Obstet Gynecol 49(2):257–269
Wang J, Chen L, Li D, Yin Y, Wang X, Li P, Dangott LJ, Hu W, Wu G (2008) Intrauterine growth restriction affects the proteomes of the small intestine, liver, and skeletal muscle in newborn pigs. J Nutr 138(1):60–66
Wang T, Huo YJ, Shi F, Xu RJ, Hutz RJ (2005) Effects of intrauterine growth retardation on development of the gastrointestinal tract in neonatal pigs. Biol Neonate 88(1):66–72. doi:10.1159/000084645
Longo S, Bollani L, Decembrino L, Di Comite A, Angelini M, Stronati M (2013) Short-term and long-term sequelae in intrauterine growth retardation (IUGR). J Matern Fetal Neonatal Med 26(3):222–225. doi:10.3109/14767058.2012.715006
Han F, Hu L, Xuan Y, Ding X, Luo Y, Bai S, He S, Zhang K, Che L (2013) Effects of high nutrient intake on the growth performance, intestinal morphology and immune function of neonatal intra-uterine growth-retarded pigs. Br J Nutr 110(10):1819–1827. doi:10.1017/S0007114513001232
Hu L, Liu Y, Yan C, Peng X, Xu Q, Xuan Y, Han F, Tian G, Fang Z, Lin Y, Xu S, Zhang K, Chen D, Wu D, Che L (2015) Postnatal nutritional restriction affects growth and immune function of piglets with intra-uterine growth restriction. Br J Nutr 114(1):53–62. doi:10.1017/S0007114515001579
Zhong X, Wang T, Zhang X, Li W (2010) Heat shock protein 70 is upregulated in the intestine of intrauterine growth retardation piglets. Cell Stress Chaperones 15(3):335–342. doi:10.1007/s12192-009-0148-3
Wang M, Radlowski EC, Monaco MH, Fahey GC Jr, Gaskins HR, Donovan SM (2013) Mode of delivery and early nutrition modulate microbial colonization and fermentation products in neonatal piglets. J Nutr 143(6):795–803. doi:10.3945/jn.112.173096
Fanca-Berthon P, Hoebler C, Mouzet E, David A, Michel C (2010) Intrauterine growth restriction not only modifies the cecocolonic microbiota in neonatal rats but also affects its activity in young adult rats. J Pediatr Gastroenterol Nutr 51(4):402–413. doi:10.1097/MPG.0b013e3181d75d52
D’Inca R, Kloareg M, Gras-Le Guen C, Le Huerou-Luron I (2010) Intrauterine growth restriction modifies the developmental pattern of intestinal structure, transcriptomic profile, and bacterial colonization in neonatal pigs. J Nutr 140(5):925–931. doi:10.3945/jn.109.116822
Lin H-C, Su B-H, Chen A-C, Lin T-W, Tsai C-H, Yeh T-F, Oh W (2005) Oral probiotics reduce the incidence and severity of necrotizing enterocolitis in very low birth weight infants. Pediatrics 115(1):1–4
Alexopoulos C, Georgoulakis IE, Tzivara A, Kyriakis CS, Govaris A, Kyriakis SC (2004) Field evaluation of the effect of a probiotic-containing Bacillus licheniformis and Bacillus subtilis spores on the health status, performance, and carcass quality of grower and finisher pigs. J Vet Med A Physiol Pathol Clin Med 51(6):306–312. doi:10.1111/j.1439-0442.2004.00637.x
Braga TD, da Silva GA, de Lira PI, de Carvalho Lima M (2011) Efficacy of Bifidobacterium breve and Lactobacillus casei oral supplementation on necrotizing enterocolitis in very-low-birth-weight preterm infants: a double-blind, randomized, controlled trial. Am J Clin Nutr 93(1):81–86. doi:10.3945/ajcn.2010.29799
Han GQ, Xiang ZT, Yu B, Chen DW, Qi HW, Mao XB, Chen H, Mao Q, Huang ZQ (2012) Effects of different starch sources on Bacillus spp. in intestinal tract and expression of intestinal development related genes of weanling piglets. Mol Biol Rep 39(2):1869–1876. doi:10.1007/s11033-011-0932-x
Hong HA, le Duc H, Cutting SM (2005) The use of bacterial spore formers as probiotics. FEMS Microbiol Rev 29(4):813–835. doi:10.1016/j.femsre.2004.12.001
Hu Y, Dun Y, Li S, Zhao S, Peng N, Liang Y (2014) Effects of Bacillus subtilis KN-42 on growth performance, diarrhea and faecal bacterial flora of weaned piglets. Asian Australas J Anim Sci 27(8):1131
Macfarlane GT, Cummings JH (1999) Probiotics and prebiotics: can regulating the activities of intestinal bacteria benefit health? BMJ 318(7189):999–1003. doi:10.1136/bmj.318.7189.999
Rolfe RD (2000) The role of probiotic cultures in the control of gastrointestinal health. J Nutr 130(2S Suppl):396S–402S
Sangild PT (2006) Gut responses to enteral nutrition in preterm infants and animals. Exp Biol Med 231(11):1695–1711
Che L, Thymann T, Bering SB, LEH-L I, D’Inca R, Zhang K, Sangild PT (2010) IUGR does not predispose to necrotizing enterocolitis or compromise postnatal intestinal adaptation in preterm pigs. Pediatr Res 67(1):54–59. doi:10.1203/PDR.0b013e3181c1b15e
Zhang Y, Chen DW, Yu B, He J, Yu J, Mao XB, Wang JX, Luo JQ, Huang ZQ, Cheng GX, Zheng P (2015) Spray-dried chicken plasma improves intestinal digestive function and regulates intestinal selected microflora in weaning piglets. J Anim Sci 93(6):2967–2976. doi:10.2527/jas.2014-8820
Chen Y, Chen D, Tian G, He J, Mao X, Mao Q, Yu B (2012) Dietary arginine supplementation alleviates immune challenge induced by Salmonella enterica serovar Choleraesuis bacterin potentially through the Toll-like receptor 4-myeloid differentiation factor 88 signalling pathway in weaned piglets. Br J Nutr 108(6):1069–1076. doi:10.1017/S0007114511006350
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25(4):402–408. doi:10.1006/meth.2001.1262
Zhang B, Guo Y (2009) Supplemental zinc reduced intestinal permeability by enhancing occludin and zonula occludens protein-1 (ZO-1) expression in weaning piglets. Br J Nutr 102(5):687–693. doi:10.1017/S0007114509289033
Garite TJ, Clark R, Thorp JA (2004) Intrauterine growth restriction increases morbidity and mortality among premature neonates. Am J Obstet Gynecol 191(2):481–487
Mandruzzato G, Antsaklis A, Botet F, Chervenak FA, Figueras F, Grunebaum A, Puerto B, Skupski D, Stanojevic M, WAPM (2008) Intrauterine restriction (IUGR). J Perinat Med 36(4):277–281. doi:10.1515/JPM.2008.050
Musa H, Wu S, Zhu C, Seri H, Zhu G (2009) The potential benefits of probiotics in animal production and health. J Anim Vet Adv 8(2):313–321
Rehfeldt C, Kuhn G (2006) Consequences of birth weight for postnatal growth performance and carcass quality in pigs as related to myogenesis. J Anim Sci 84(Suppl):E113–E123
Morise A, Seve B, Mace K, Magliola C, Le Huerou-Luron I, Louveau I (2011) Growth, body composition and hormonal status of growing pigs exhibiting a normal or small weight at birth and exposed to a neonatal diet enriched in proteins. Br J Nutr 105(10):1471–1479. doi:10.1017/S0007114510005386
Alvarenga AL, Chiarini-Garcia H, Cardeal PC, Moreira LP, Foxcroft GR, Fontes DO, Almeida FR (2013) Intra-uterine growth retardation affects birthweight and postnatal development in pigs, impairing muscle accretion, duodenal mucosa morphology and carcass traits. Reprod Fertil Dev 25(2):387–395. doi:10.1071/RD12021
Chen Y, Min B, Cho J, Kwon O, Son K, Kim H, Kim I (2006) Effects of dietary Bacillus-based probiotic on growth performance, nutrients digestibility, blood characteristics and fecal noxious gas content in finishing pigs. Asian Australas J Anim Sci 19(4):587
Lee SH, Ingale SL, Kim JS, Kim KH, Lokhande A, Kim EK, Kwon IK, Kim YH, Chae BJ (2014) Effects of dietary supplementation with Bacillus subtilis LS 1–2 fermentation biomass on growth performance, nutrient digestibility, cecal microbiota and intestinal morphology of weanling pig. Anim Feed Sci Technol 188:102–110. doi:10.1016/j.anifeedsci.2013.12.001
Fanca-Berthon P, Michel C, Pagniez A, Rival M, Van Seuningen I, Darmaun D, Hoebler C (2009) Intrauterine growth restriction alters postnatal colonic barrier maturation in rats. Pediatr Res 66(1):47–52. doi:10.1203/PDR.0b013e3181a2047e
Amdi C, Krogh U, Flummer C, Oksbjerg N, Hansen CF, Theil PK (2013) Intrauterine growth restricted piglets defined by their head shape ingest insufficient amounts of colostrum. J Anim Sci 91(12):5605–5613. doi:10.2527/jas.2013-6824
Hales J, Moustsen VA, Nielsen MB, Hansen CF (2013) Individual physical characteristics of neonatal piglets affect preweaning survival of piglets born in a noncrated system. J Anim Sci 91(10):4991–5003. doi:10.2527/jas.2012-5740
Garite TJ, Clark R, Thorp JA (2004) Intrauterine growth restriction increases morbidity and mortality among premature neonates. Am J Obstet Gynecol 191(2):481–487. doi:10.1016/j.ajog.2004.01.036
Dong L, Zhong X, He J, Zhang L, Bai K, Xu W, Wang T, Huang X (2015) Supplementation of tributyrin improves the growth and intestinal digestive and barrier functions in intrauterine growth-restricted piglets. Clin Nutr. doi:10.1016/j.clnu.2015.03.002
Xu RJ, Mellor DJ, Birtles MJ, Reynolds GW, Simpson HV (1994) Impact of intrauterine growth retardation on the gastrointestinal tract and the pancreas in newborn pigs. J Pediatr Gastroenterol Nutr 18(2):231–240
Montagne L, Pluske J, Hampson D (2003) A review of interactions between dietary fibre and the intestinal mucosa, and their consequences on digestive health in young non-ruminant animals. Anim Feed Sci Technol 108(1):95–117
Kelly D, Coutts AG (2000) Early nutrition and the development of immune function in the neonate. Proc Nutr Soc 59(2):177–185
Amu S, Hahn-Zoric M, Malik A, Ashraf R, Zaman S, Kjellmer I, Hagberg H, Padyukov L, Hanson LÅ (2006) Cytokines in the placenta of Pakistani newborns with and without intrauterine growth retardation. Pediatr Res 59(2):254–258
Briana DD, Liosi S, Gourgiotis D, Boutsikou M, Marmarinos A, Baka S, Hassiakos D, Malamitsi-Puchner A (2012) Fetal concentrations of the growth factors TGF-alpha and TGF-beta1 in relation to normal and restricted fetal growth at term. Cytokine 60(1):157–161. doi:10.1016/j.cyto.2012.06.005
Contreras YM, Yu X, Hale MA, Callaway CW, Bareyan D, McKnight RA, Joss-Moore LA, Enioutina EY, Lane RH (2011) Intrauterine growth restriction alters T-lymphocyte cell number and dual specificity phosphatase 1 levels in the thymus of newborn and juvenile rats. Pediatr Res 70(2):123–129. doi:10.1203/PDR.0b013e31821f6e75
Morrison JL (2008) Sheep models of intrauterine growth restriction: fetal adaptations and consequences. Clin Exp Pharmacol Physiol 35(7):730–743. doi:10.1111/j.1440-1681.2008.04975.x
Wang S-P, Yang L, Tang X-S, Cai L-C, Liu G, Kong X-F, Blachier F, Yin Y-L (2011) Dietary supplementation with high-dose Bacillus subtilis or Lactobacillus reuteri modulates cellular and humoral immunities and improves performance in weaned piglets. J Food Agric Environ 9(2):181–187
Suarez-Souto MA, Lara-Padilla E, Reyna-Garfias H, Viloria M, Lopez-Sanchez P, Rivera-Aguilar V, Miliar-Garcia A, Kormanovski A, Dominguez-Lopez ML, Campos-Rodriguez R (2012) Caloric restriction modifies both innate and adaptive immunity in the mouse small intestine. J Physiol Biochem 68(2):163–173. doi:10.1007/s13105-011-0128-9
Newburg DS, Walker WA (2007) Protection of the neonate by the innate immune system of developing gut and of human milk. Pediatr Res 61(1):2–8. doi:10.1203/01.pdr.0000250274.68571.18
Borchers AT, Selmi C, Meyers FJ, Keen CL, Gershwin ME (2009) Probiotics and immunity. J Gastroenterol 44(1):26–46. doi:10.1007/s00535-008-2296-0
Lalles JP (2012) Long term effects of pre- and early postnatal nutrition and environment on the gut. J Anim Sci 90(Suppl 4):421–429. doi:10.2527/jas.53904
Elkouby-Naor L, Ben-Yosef T (2010) Functions of claudin tight junction proteins and their complex interactions in various physiological systems. Int Rev Cell Mol Biol 279:1–32. doi:10.1016/S1937-6448(10)79001-8
Blasig IE, Bellmann C, Cording J, Del Vecchio G, Zwanziger D, Huber O, Haseloff RF (2011) Occludin protein family: oxidative stress and reducing conditions. Antioxid Redox Signal 15(5):1195–1219. doi:10.1089/ars.2010.3542
Acknowledgments
The present study was supported by the National Natural Science Foundation of China (31101727); the International Cooperation in Science and Technology Project of Sichuan Province (2014HH0034); the Program for Changjiang Scholars and Innovative Research Team in University (IRT13083); and the Natural Science Foundation of Sichuan Province (12ZA110).
Author contributions
The authors’ contributions are as follows: L. H. and L. C. designed the study; L. H., X. P., C. Y., Y. L. and Q. X. carried out the study; L. H., L. Q., Z. F., Y. L., S. X., B. F., J. L. and D. W. performed the analysis and analysed the data; L. H. wrote the paper; and L. C. and H. C. made some modifications in the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Rights and permissions
About this article
Cite this article
Hu, L., Peng, X., Chen, H. et al. Effects of intrauterine growth retardation and Bacillus subtilis PB6 supplementation on growth performance, intestinal development and immune function of piglets during the suckling period. Eur J Nutr 56, 1753–1765 (2017). https://doi.org/10.1007/s00394-016-1223-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-016-1223-z