Abstract
Purpose
Hedonic eating occurs independently from homeostatic needs prompting the ingestion of pleasurable foods that are typically rich in fat, sugar and/or salt content. In normal weight healthy subjects, we found that before hedonic eating, plasma levels of 2-arachidonoylglycerol (2-AG) were higher than before nonhedonic eating, and although they progressively decreased after food ingestion in both eating conditions, they were significantly higher in hedonic eating. Plasma levels of anandamide (AEA), oleoylethanolamide (OEA) and palmitoylethanolamide (PEA), instead, progressively decreased in both eating conditions without significant differences. In this study, we investigated the responses of AEA, 2-AG, OEA and PEA to hedonic eating in obese individuals.
Methods
Peripheral levels of AEA, 2-AG, OEA and PEA were measured in 14 obese patients after eating favourite (hedonic eating) and non-favourite (nonhedonic eating) foods in conditions of no homeostatic needs.
Results
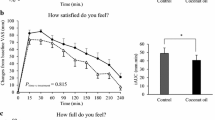
Plasma levels of 2-AG increased after eating the favourite food, whereas they decreased after eating the non-favourite food, with the production of the endocannabinoid being significantly enhanced in hedonic eating. Plasma levels of AEA decreased progressively in nonhedonic eating, whereas they showed a decrease after the exposure to the favourite food followed by a return to baseline values after eating it. No significant differences emerged in plasma OEA and PEA responses to favourite and non-favourite food.
Conclusion
Present findings compared with those obtained in our previously studied normal weight healthy subjects suggest deranged responses of endocannabinoids to food-related reward in obesity.


Similar content being viewed by others
References
Lowe MR, Butryn ML (2007) Hedonic hunger: a new dimension of appetite? Physiol Behav 91:432–439
Nummenmaa L, Hirvonen J, Hannukainen J, Immonen H, Lindroos M, Salminen P, Nuutila P (2012) Dorsal striatum and its limbic connectivity mediate abnormal anticipatory reward processing in obesity. PLoS One 7:e31089–e31089
Swinburn B, Sacks G, Hall K, McPherson K, Finegood D, Moodie ML, Gortmaker SL (2011) The global obesity pandemic: shaped by global drivers and local environments. Lancet 378:804–814
Volkow N, Wang G, Tomasi D, Baler R (2013) The addictive dimensionality of obesity. Biol Psychiatry 73:811–818
Koob G, Volkow N (2010) Neurocircuitry of addiction. Neuropsychopharmacology 35:217–238
Di Marzo V (2008) Targeting the endocannabinoid system: to enhance or reduce? Nat Rev Drug Discov 7:438–455
Cristino L, Becker T, Di Marzo V (2014) Endocannabinoids and energy homeostasis: an update. Biofactors 40:389–397
Lo Verme J, Gaetani S, Fu J, Oveisi F, Burton K, Piomelli D (2005) Regulation of food intake by oleoylethanolamide. Cell Mol Life Sci 62:708–716
Movahed P, Jönsson BA, Birnir B, Wingstrand JA, Jørgensen TD, Ermund A, Sterner O, Zygmunt PM, Högestätt ED (2005) Endogenous unsaturated C18 N-acylethanolamines are vanilloid receptor (TRPV1) agonists. J Biol Chem 280:38496–38504
Silvestri C, Di Marzo V (2013) The endocannabinoid system in energy homeostasis and the etiopathology of metabolic disorders. Cell Metab 17:475–490
Adamczyk P, Miszkiel J, McCreary AC, Filip M, Papp M, Przegaliński E (2012) The effects of cannabinoid CB1, CB2 and vanilloid TRPV1 receptor antagonists on cocaine addictive behavior in rats. Brain Res 1444:45–54
Melis M, Muntoni AL, Pistis M (2012) Endocannabinoids and the processing of value-related signals. Front Pharmacol 3:7
Monteleone P, Matias I, Martiadis V, De Petrocellis L, Maj M, Di Marzo V (2005) Blood levels of the endocannabinoid anandamide are increased in anorexia nervosa and in binge-eating disorder, but not in bulimia nervosa. Neuropsychopharmacology 30:1216–1221
Matias I, Gonthier MP, Orlando P, Martiadis V, De Petrocellis L, Cervino C, Petrosino S, Hoareau L, Festy F, Pasquali R, Roche R, Maj M, Pagotto U, Monteleone P, Di Marzo V (2006) Regulation, function, and dysregulation of endocannabinoids in models of adipose and beta-pancreatic cells and in obesity and hyperglycemia. J Clin Endocrinol Metab 91:3171–3180
Monteleone P, Piscitelli F, Scognamiglio P, Monteleone AM, Canestrelli B, Di Marzo V, Maj M (2012) Hedonic eating is associated with increased peripheral levels of ghrelin and the endocannabinoid 2-arachidonoyl-glycerol in healthy humans: a pilot study. J Clin Endocrinol Metab 97:E917–E924
World Health Organization (1998) Obesity: preventing and managing the global epidemic: report of a WHO consultation on obesity (WHO/NUT/NCD). WHO, Geneva
Annuzzi G, Piscitelli F, Di Marino L, Patti L, Giacco R, Costabile G, Bozzetto L, Riccardi G, Verde R, Petrosino S, Rivellese AA, Di Marzo V (2010) Differential alterations of the concentrations of endocannabinoids and related lipids in the subcutaneous adipose tissue of obese diabetic patients. Lipids Health Dis 9:43
Dixon JW (1992) BMDP statistical software. Release 7.0. University of California Press, Berkeley
Melis T, Succu S, Sanna F, Boi A, Argiolas A, Melis MR (2007) The cannabinoid antagonist SR 141716A (Rimonabant) reduces the increase of extra-cellular dopamine release in the rat nucleus accumbens induced by a novel high palatable food. Neurosci Lett 419:231–235
Di Marzo V (2011) Endocannabinoids: an appetite for fat. Proc Natl Acad Sci USA 108:12567–12568
Piomelli D (2013) A fatty gut feeling. Trends Endocrinol Metab 24:332–341
Erlanson-Albertsson C, Lindqvist A (2010) Fructose affects enzymes involved in the synthesis and degradation of hypothalamic endocannabinoids. Regul Pept 161:87–91
Di Chiara G (2000) Role of dopamine in the behavioural actions of nicotine related to addiction. Eur J Pharmacol 393:295–314
Burger KS, Stice E (2012) Frequent ice cream consumption is associated with reduced striatal response to receipt of an ice cream-based milkshake. Am J Clin Nutr 95:810–817
Avena NM, Rada P, Hoebel BG (2008) Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci Biobehav Rev 32:20–39
Dagher A (2009) The neurobiology of appetite: hunger as addiction. Int J Obes (Lond) 33(Suppl 2):S30–S33
Ziauddeen H, Farooqi IS, Fletcher PC (2012) Obesity and the brain: how convincing is the addiction model? Nat Rev Neurosci 13:279–286
Mahler SV, Smith KS, Berridge KC (2007) Endocannabinoid hedonic hotspot for sensory pleasure: anandamide in nucleus accumbens shell enhances ‘liking’ of a sweet reward. Neuropsychopharmacology 32:2267–2278
Smith KS, Mahler SV, Pecina S, Berridge KC (2010) Hedonic hotspots: generating sensory pleasure in the brain. In: Kringelbach ML, Berridge KC (eds) Pleasures of the Brain. Oxford University Press, Oxford, pp 27–49
Dalton M, Blundell J, Finlayson GS (2013) Examination of food reward and energy intake under laboratory and free-living conditions in a trait binge eating subtype of obesity. Front Psychol 4:1–8
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical standards
All the participants signed a written informed consent; the study was approved by the Ethics Committee of the University of Naples SUN; all the procedures were in accordance with the ethical standards laid down by the 1964 Declaration of Helsinki and its later amendments.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Monteleone, A.M., Di Marzo, V., Monteleone, P. et al. Responses of peripheral endocannabinoids and endocannabinoid-related compounds to hedonic eating in obesity. Eur J Nutr 55, 1799–1805 (2016). https://doi.org/10.1007/s00394-016-1153-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-016-1153-9