Abstract
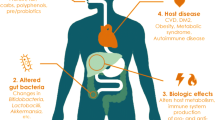
The gut microbiota has been extensively studied in all health science fields because its imbalance is linked to many disorders, such as inflammation and oxidative stress, thereby contributing to cardiovascular disease, obesity, diabetes and chronic kidney disease (CKD) complications. Novel therapeutic strategies that aim to reduce the complications caused by this imbalance have increased in recent years. Studies have shown that prebiotic supplementation can beneficially modulate the gut microbiota in CKD patients. Prebiotics consist of non-digestible dietary soluble fiber, which acts as a substrate for the gut microbiota. Resistant starch (RS) is a type of dietary fiber that can reach the large bowel and act as a substrate for microbial fermentation; for these reasons, it has been considered to be a prebiotic. Few studies have analyzed the effects of RS on the gut microbiota in CKD patients. This review discusses recent information about RS and the potential role of the gut microbiota, with a particular emphasis on CKD patients.

Similar content being viewed by others
References
Mafra D, Fouque D (2015) Gut microbiota and inflammation in chronic kidney disease patients. Clin Kidney J. doi:10.1093/ckj/sfv026
Mafra D, Lobo JC, Barros AF et al (2014) Role of altered intestinal microbiota in systemic inflammation and cardiovascular disease in chronic kidney disease. Future Microbiol 9:399–410. doi:10.2217/fmb.13.165
Le Chatelier E, Nielsen T, Qin J et al (2013) Richness of human gut microbiome correlates with metabolic markers. Nature 500:541–546. doi:10.1038/nature12506
Kasubuchi M, Hasegawa S, Hiramatsu T et al (2015) Dietary gut microbial metabolites, short-chain fatty acids, and host metabolic regulation. Nutrients 7:2839–2849. doi:10.3390/nu7042839
Vaziri ND, Wong J, Pahl M et al (2013) Chronic kidney disease alters intestinal microbial flora. Kidney Int 83:308–315. doi:10.1038/ki.2012.345
Barros AF, Borges NA, Ferreira DC et al (2015) Is there interaction between gut microbial profile and cardiovascular risk in chronic kidney disease patients? Future Microbiol 10:517–526. doi:10.2217/fmb.14.140
Vanholder R, Glorieux G (2015) The intestine and the kidneys: a bad marriage can be hazardous. Clin Kidney J 8:168–179. doi:10.1093/ckj/sfv004
Ranganathan N, Patel B, Ranganathan P et al (2005) Probiotic amelioration of azotemia in 5/6th nephrectomized Sprague-Dawley rats. Sci World J 5:652–660. doi:10.1100/tsw.2005.86
Hyun HS, Paik KH, Cho HY (2013) p-Cresyl sulfate and indoxyl sulfate in pediatric patients on chronic dialysis. Korean J Pediatr 56:159–164. doi:10.3345/kjp.2013.56.4.159
Vaziri ND, Zhao Y-Y, Pahl MV (2015) Altered intestinal microbial flora and impaired epithelial barrier structure and function in CKD: the nature, mechanisms, consequences and potential treatment. Nephrol Dial Transpl. doi:10.1093/ndt/gfv095
Laetitia K, Denise M, Dennis F (2015) Probiotics and chronic kidney disease. Kidney Int 88(5):958–966. doi:10.1038/ki.2015.255
Vaziri ND, Zhao Y-Y, Pahl MV (2015) Altered intestinal microbial flora and impaired epithelial barrier structure and function in CKD: the nature, mechanisms, consequences and potential treatment. Nephrol Dial Transpl Off Publ Eur Dial Transpl Assoc-Eur Ren Assoc. doi:10.1093/ndt/gfv095
Cummings JH, Stephen AM (2007) Carbohydrate terminology and classification. Eur J Clin Nutr 61(Suppl 1):S5–18. doi:10.1038/sj.ejcn.1602936
Topping DL, Clifton PM (2001) Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev 81:1031–1064
Alimentarius Codex (2010) Guidelines on nutrition labelling CAC/GL 2-1985 as last amended 2010. Joint FAO/WHO Food Standards Programme, Secretariat of the Codex Alimentarius Commission, FAO, Rome
Englyst HN, Kingman SM, Cummings JH (1992) Classification and measurement of nutritionally important starch fractions. Eur J Clin Nutr 46(Suppl 2):S33–50
Topping DL, Fukushima M, Bird AR (2003) Resistant starch as a prebiotic and synbiotic: state of the art. Proc Nutr Soc 62:171–176
Case SE, Capitani T, Whaley JK et al (1998) Physical properties and gelation behavior of a low-amylopectin maize starch and other high-amylose maize starches. J Cereal Sci 27:301–314
Jiang H, Lio J, Blanco M et al (2010) Resistant-starch formation in high-amylose maize starch during kernel development. J Agric Food Chem 58:8043–8047. doi:10.1021/jf101056y
Shi YC, Capitani T, Trzasco P, Jeffcoat R (1998) Molecular structure of a low-amylopectin starch and other high-amylose maize starches. J Cereal Sci 27:289–299
Englyst H, Wiggins HS, Cummings JH (1982) Determination of the non-starch polysaccharides in plant foods by gas–liquid chromatography of constituent sugars as alditol acetates. The Analyst 107:307–318
Englyst HN, Kingman SM, Hudson GJ, Cummings JH (1996) Measurement of resistant starch in vitro and in vivo. Br J Nutr 75:749–755
Brown MA, Storlien LH, Brown IL, Higgins JA (2003) Cooking attenuates the ability of high-amylose meals to reduce plasma insulin concentrations in rats. Br J Nutr 90:823–827
Bird AR, Hayakawa T, Marsono Y et al (2000) Coarse brown rice increases fecal and large bowel short-chain fatty acids and starch but lowers calcium in the large bowel of pigs. J Nutr 130:1780–1787
McBurney MI (1991) Passage of starch into the colon of humans: quantitation and implications. Can J Physiol Pharmacol 69:130–136
Keenan MJ, Zhou J, Hegsted M et al (2015) Role of resistant starch in improving gut health, adiposity, and insulin resistance. Adv Nutr Bethesda Md 6:198–205. doi:10.3945/an.114.007419
Panesar PS, Kumari S, Panesar R (2013) Biotechnological approaches for the production of prebiotics and their potential applications. Crit Rev Biotechnol 33:345–364. doi:10.3109/07388551.2012.709482
Zaman SA, Sarbini SR (2015) The potential of resistant starch as a prebiotic. Crit Rev Biotechnol. doi:10.3109/07388551.2014.993590
Murphy MM, Douglass JS, Birkett A (2008) Resistant starch intakes in the United States. J Am Diet Assoc 108:67–78. doi:10.1016/j.jada.2007.10.012
Asp NG, van Amelsvoort JM, Hautvast JG (1996) Nutritional implications of resistant starch. Nutr Res Rev 9:1–31. doi:10.1079/NRR19960004
Monsivais P, Carter BE, Christiansen M et al (2011) Soluble fiber dextrin enhances the satiating power of beverages. Appetite 56:9–14. doi:10.1016/j.appet.2010.10.010
Lobley GE, Holtrop G, Bremner DM et al (2013) Impact of short term consumption of diets high in either non-starch polysaccharides or resistant starch in comparison with moderate weight loss on indices of insulin sensitivity in subjects with metabolic syndrome. Nutrients 5:2144–2172. doi:10.3390/nu5062144
Salonen A, Lahti L, Salojärvi J et al (2014) Impact of diet and individual variation on intestinal microbiota composition and fermentation products in obese men. ISME J 8:2218–2230. doi:10.1038/ismej.2014.63
Sarbini SR, Kolida S, Deaville ER et al (2014) Potential of novel dextran oligosaccharides as prebiotics for obesity management through in vitro experimentation. Br J Nutr 112:1303–1314. doi:10.1017/S0007114514002177
Sleeth ML, Thompson EL, Ford HE et al (2010) Free fatty acid receptor 2 and nutrient sensing: a proposed role for fibre, fermentable carbohydrates and short-chain fatty acids in appetite regulation. Nutr Res Rev 23:135–145. doi:10.1017/S0954422410000089
Bach Knudsen KE (2015) Microbial degradation of whole-grain complex carbohydrates and impact on short-chain fatty acids and health. Adv Nutr Bethesda Md 6:206–213. doi:10.3945/an.114.007450
Hoeppli RE, Wu D, Cook L, Levings MK (2015) The environment of regulatory T cell biology: cytokines, metabolites, and the microbiome. Front Immunol 6:61. doi:10.3389/fimmu.2015.00061
Cummings JH, Pomare EW, Branch WJ et al (1987) Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 28:1221–1227
Denise Robertson M (2007) Metabolic cross talk between the colon and the periphery: implications for insulin sensitivity. Proc Nutr Soc 66:351–361. doi:10.1017/S0029665107005617
Zhou Z, Wang F, Ren X et al (2015) Resistant starch manipulated hyperglycemia/hyperlipidemia and related genes expression in diabetic rats. Int J Biol Macromol 75:316–321. doi:10.1016/j.ijbiomac.2015.01.052
Shen L, Keenan MJ, Martin RJ et al (2009) Dietary resistant starch increases hypothalamic POMC expression in rats. Obes Silver Spring Md 17:40–45. doi:10.1038/oby.2008.483
Nichenametla SN, Weidauer LA, Wey HE et al (2014) Resistant starch type 4-enriched diet lowered blood cholesterols and improved body composition in a double blind controlled cross-over intervention. Mol Nutr Food Res 58:1365–1369. doi:10.1002/mnfr.201300829
Johansson EV, Nilsson AC, Östman EM, Björck IME (2013) Effects of indigestible carbohydrates in barley on glucose metabolism, appetite and voluntary food intake over 16 h in healthy adults. Nutr J 12:46. doi:10.1186/1475-2891-12-46
Ble-Castillo JL, Aparicio-Trápala MA, Francisco-Luria MU et al (2010) Effects of native banana starch supplementation on body weight and insulin sensitivity in obese Type 2 diabetics. Int J Environ Res Public Health 7:1953–1962. doi:10.3390/ijerph7051953
Sirich TL, Plummer NS, Gardner CD et al (2014) Effect of increasing dietary fiber on plasma levels of colon-derived solutes in hemodialysis patients. Clin J Am Soc Nephrol CJASN 9:1603–1610. doi:10.2215/CJN.00490114
Vaziri ND, Liu S-M, Lau WL et al (2014) High amylose resistant starch diet ameliorates oxidative stress, inflammation, and progression of chronic kidney disease. PLoS ONE 9:e114881. doi:10.1371/journal.pone.0114881
Poquette NM, Gu X, Lee S-O (2014) Grain sorghum muffin reduces glucose and insulin responses in men. Food Funct 5:894–899. doi:10.1039/c3fo60432b
Bodinham CL, Al-Mana NM, Smith L, Robertson MD (2013) Endogenous plasma glucagon-like peptide-1 following acute dietary fibre consumption. Br J Nutr 110:1429–1433. doi:10.1017/S0007114513000731
Ekström LMNK, Björck IME, Ostman EM (2013) On the possibility to affect the course of glycaemia, insulinaemia, and perceived hunger/satiety to bread meals in healthy volunteers. Food Funct 4:522–529. doi:10.1039/c2fo30251a
Klosterbuer AS, Hullar MAJ, Li F et al (2013) Gastrointestinal effects of resistant starch, soluble maize fibre and pullulan in healthy adults. Br J Nutr 110:1068–1074. doi:10.1017/S0007114513000019
Robertson MD, Wright JW, Loizon E et al (2012) Insulin-sensitizing effects on muscle and adipose tissue after dietary fiber intake in men and women with metabolic syndrome. J Clin Endocrinol Metab 97:3326–3332. doi:10.1210/jc.2012-1513
Kwak JH, Paik JK, Kim HI et al (2012) Dietary treatment with rice containing resistant starch improves markers of endothelial function with reduction of postprandial blood glucose and oxidative stress in patients with prediabetes or newly diagnosed type 2 diabetes. Atherosclerosis 224:457–464. doi:10.1016/j.atherosclerosis.2012.08.003
Klosterbuer AS, Thomas W, Slavin JL (2012) Resistant starch and pullulan reduce postprandial glucose, insulin, and GLP-1, but have no effect on satiety in healthy humans. J Agric Food Chem 60:11928–11934. doi:10.1021/jf303083r
Bodinham CL, Smith L, Wright J et al (2012) Dietary fibre improves first-phase insulin secretion in overweight individuals. PLoS ONE 7:e40834. doi:10.1371/journal.pone.0040834
Maki KC, Pelkman CL, Finocchiaro ET et al (2012) Resistant starch from high-amylose maize increases insulin sensitivity in overweight and obese men. J Nutr 142:717–723. doi:10.3945/jn.111.152975
Clarke JM, Topping DL, Christophersen CT et al (2011) Butyrate esterified to starch is released in the human gastrointestinal tract. Am J Clin Nutr 94:1276–1283. doi:10.3945/ajcn.111.017228
Burn J, Bishop DT, Chapman PD et al (2011) A randomized placebo-controlled prevention trial of aspirin and/or resistant starch in young people with familial adenomatous polyposis. Cancer Prev Res Phila Pa 4:655–665. doi:10.1158/1940-6207.CAPR-11-0106
Johnston KL, Thomas EL, Bell JD et al (2010) Resistant starch improves insulin sensitivity in metabolic syndrome. Diabet Med J Br Diabet Assoc 27:391–397. doi:10.1111/j.1464-5491.2010.02923.x
Penn-Marshall M, Holtzman GI, Holtzman GI, Barbeau WE (2010) African Americans may have to consume more than 12 g a day of resistant starch to lower their risk for type 2 diabetes. J Med Food 13:999–1004. doi:10.1089/jmf.2009.0195
Li M, Piao J-H, Tian Y et al (2010) Postprandial glycaemic and insulinaemic responses to GM-resistant starch-enriched rice and the production of fermentation-related H2 in healthy Chinese adults. Br J Nutr 103:1029–1034. doi:10.1017/S0007114509992820
Martínez I, Kim J, Duffy PR et al (2010) Resistant starches Types 2 and 4 Have differential effects on the composition of the fecal microbiota in human subjects. PLoS ONE. doi:10.1371/journal.pone.0015046
Stewart ML, Nikhanj SD, Timm DA et al (2010) Evaluation of the effect of four fibers on laxation, gastrointestinal tolerance and serum markers in healthy humans. Ann Nutr Metab 56:91–98. doi:10.1159/000275962
Verbeke K, Ferchaud-Roucher V, Preston T et al (2010) Influence of the type of indigestible carbohydrate on plasma and urine short-chain fatty acid profiles in healthy human volunteers. Eur J Clin Nutr 64:678–684. doi:10.1038/ejcn.2010.92
Maki KC, Sanders LM, Reeves MS et al (2009) Beneficial effects of resistant starch on laxation in healthy adults. Int J Food Sci Nutr 60(Suppl 4):296–305. doi:10.1080/09637480903130538
Worthley DL, Le Leu RK, Whitehall VL et al (2009) A human, double-blind, placebo-controlled, crossover trial of prebiotic, probiotic, and synbiotic supplementation: effects on luminal, inflammatory, epigenetic, and epithelial biomarkers of colorectal cancer. Am J Clin Nutr 90:578–586. doi:10.3945/ajcn.2009.28106
Krishnamurthy VMR, Wei G, Baird BC et al (2012) High dietary fiber intake is associated with decreased inflammation and all-cause mortality in patients with chronic kidney disease. Kidney Int 81:300–306. doi:10.1038/ki.2011.355
Younes H, Egret N, Hadj-Abdelkader M et al (2006) Fermentable carbohydrate supplementation alters nitrogen excretion in chronic renal failure. J Ren Nutr Off J Counc Ren Nutr Natl Kidney Found 16:67–74. doi:10.1053/j.jrn.2005.10.007
Chiavaroli L, Mirrahimi A, Sievenpiper JL et al (2015) Dietary fiber effects in chronic kidney disease: a systematic review and meta-analysis of controlled feeding trials. Eur J Clin Nutr 69:761–768. doi:10.1038/ejcn.2014.237
Hooper LV, Midtvedt T, Gordon JI (2002) How host-microbial interactions shape the nutrient environment of the mammalian intestine. Annu Rev Nutr 22:283–307. doi:10.1146/annurev.nutr.22.011602.092259
Meijers BKI, De Preter V, Verbeke K et al (2010) p-Cresyl sulfate serum concentrations in haemodialysis patients are reduced by the prebiotic oligofructose-enriched inulin. Nephrol Dial Transpl Off Publ Eur Dial Transpl Assoc - Eur Ren Assoc 25:219–224. doi:10.1093/ndt/gfp414
Fujita H, Morii T, Fujishima H et al (2014) The protective roles of GLP-1R signaling in diabetic nephropathy: possible mechanism and therapeutic potential. Kidney Int 85:579–589. doi:10.1038/ki.2013.427
Wong J, Piceno YM, Desantis TZ et al (2014) Expansion of urease- and uricase-containing, indole- and p-cresol-forming and contraction of short-chain fatty acid-producing intestinal microbiota in ESRD. Am J Nephrol 39:230–237. doi:10.1159/000360010
Baer DJ, Stote KS, Henderson T et al (2014) The metabolizable energy of dietary resistant maltodextrin is variable and alters fecal microbiota composition in adult men. J Nutr 144:1023–1029. doi:10.3945/jn.113.185298
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare there are no conflicts of interest.
Rights and permissions
About this article
Cite this article
Moraes, C., Borges, N.A. & Mafra, D. Resistant starch for modulation of gut microbiota: Promising adjuvant therapy for chronic kidney disease patients?. Eur J Nutr 55, 1813–1821 (2016). https://doi.org/10.1007/s00394-015-1138-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-015-1138-0