Abstract
Purpose
In recent years, the increasing consumption of soft drinks containing high-fructose corn syrup or sucrose has caused a rise in fructose intake, which has been related to the epidemic of metabolic diseases. As fructose and glucose intake varies in parallel, it is still unclear what the effects of the increased consumption of the two single sugars are. In the present study, the impact of chronic consumption of glucose or fructose on skeletal muscle of healthy mice was investigated.
Methods
C57BL/6J male mice received water (C), 15 % fructose (ChF) or 15 % glucose (ChG) to drink for up to 7 months. Lipid metabolism and markers of inflammation and autophagy were assessed in gastrocnemius muscle.
Results
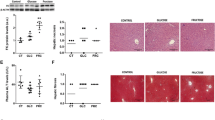
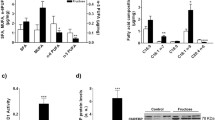
Increased body weight and gastrocnemius muscle mass, as well as circulating glucose, insulin, and lipid plasma levels were observed in sugar-drinking mice. Although triglycerides increased in the gastrocnemius muscle of both ChF and ChG mice (+32 and +26 %, vs C, respectively), intramyocellular lipids accumulated to a significantly greater extent in ChF than in ChG animals (ChF +10 % vs ChG). Such perturbations were associated with increased muscle interleukin-6 levels (threefold of C) and with the activation of autophagy, as demonstrated by the overexpression of LC3B-II (ChF, threefold and ChG, twofold of C) and beclin-1 (ChF, sevenfold and ChG, tenfold of C).
Conclusions
The present results suggest that intramyocellular lipids and the pro-inflammatory signaling could contribute to the onset of insulin resistance and lead to the induction of autophagy, which could be an adaptive response to lipotoxicity.





Similar content being viewed by others
References
Park YK, Yetley EA (1993) Intakes and food sources of fructose in the United States. Am J Clin Nutr 58(5):737S–747S
Tappy L, Lê KA (2010) Metabolic effects of fructose and the worldwide increase in obesity. Physiol Rev 90:23–46. doi:10.1152/physrev.00019.2009
Dekker MJ, Su Q, Baker C, Rutledge AC, Adeli K (2010) Fructose: a highly lipogenic nutrient implicated in insulin resistance, hepatic steatosis, and metabolic syndrome. Am J Physiol Encocrinol Metab 299:E685–E694. doi:10.1152/ajpendo.00283.2010
Tappy L, Lê KA, Tran C, Paquot N (2010) Fructose and metabolic diseases: new findings, new questions. Nutrition 26:1044–1049. doi:10.1016/j.nut.2010.02.014
Ludwig DS, Peterson KE, Gortmaker SL (2001) Relation between consumption of sugar-sweetened drinks and childhood obesity: a prospective, observational analysis. Lancet 357(9255):505–508. doi:10.1016/S0140-6736(00)04041-1
Duffey KJ, Huybrechts I, Mouratidou T, Libuda L, Kersting M, De Vriendt T, Gottrand F, Widhalm K, Dallongeville J, Hallström L, González-Gross M, De Henauw S, Moreno LA, Popkin BM, HELENA Study Group (2012) Beverage consumption among European adolescents in the HELENA study. Eur J Clin Nutr 66(2):244–252. doi:10.1038/ejcn.2011.166
Bray GA, Nielsen SJ, Popkin BM (2004) Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr 79(4):537–543
Rizkalla SW (2010) Health implications of fructose consumption: a review of recent data. Nutr Metab (Lond) 7:82. doi:10.1186/1743-7075-7-82
Mayes PA (1993) Intermediary metabolism of fructose. Am J Clin Nutr 58(5):754S–765S
Mastrocola R, Collino M, Rogazzo M, Medana C, Nigro D, Boccuzzi G, Aragno M (2013) Advanced glycation end products promote hepatosteatosis by interfering with SCAP-SREBP pathway in fructose-drinking mice. Am J Physiol Gastrointest Liver Physiol 15:G398–G407. doi:10.1152/ajpgi.00450.2012
Kazumi T, Vranic M, Steiner G (1986) Triglyceride kinetics: effects of dietary glucose, sucrose, or fructose alone or with hyperinsulinemia. Am J Physiol 250(3 Pt 1):E325–E330
Jameel F, Phang M, Wood LG, Garg ML (2014) Acute effects of feeding fructose, glucose and sucrose on blood lipid levels and systemic inflammation. Lipids Health Dis 13:195. doi:10.1186/1476-511X-13-195
Stanhope KL, Schwarz JM, Keim NL, Griffen SC, Bremer AA, Graham JL, Hatcher B, Cox CL, Dyachenko A, Zhang W, McGahan JP, Seibert A, Krauss RM, Chiu S, Schaefer EJ, Ai M, Otokozawa S, Nakajima K, Nakano T, Beysen C, Hellerstein MK, Berglund L, Havel PJ (2009) Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest. 119(5):1322–1334. doi:10.1172/JCI37385
Cox CL, Stanhope KL, Schwarz JM, Graham JL, Hatcher B, Griffen SC, Bremer AA, Berglund L, McGahan JP, Havel PJ, Keim NL (2012) Consumption of fructose-sweetened beverages for 10 weeks reduces net fat oxidation and energy expenditure in overweight/obese men and women. Eur J Clin Nutr 66(2):201–208. doi:10.1038/ejcn.2011.159
Aeberli I, Hochuli M, Gerber PA, Sze L, Murer SB, Tappy L, Spinas GA, Berneis K (2013) Moderate amounts of fructose consumption impair insulin sensitivity in healthy young men: a randomized controlled trial. Diabetes Care 36(1):150–156. doi:10.2337/dc12-0540
Moore JB, Gunn PJ, Fielding BA (2014) The role of dietary sugars and de novo lipogenesis in non-alcoholic fatty liver disease. Nutrients 6(12):5679–5703. doi:10.3390/nu6125679
Naismith DJ (1971) Differences in the metabolism of dietary carbohydrates studied in the rat. Proc Nutr Soc 30(3):259–265
Bruckdorfer KR, Khan IH, Yudkin J (1972) Fatty acid synthetase activity in the liver and adipose tissue of rats fed with various carbohydrates. Biochem J 129(2):439–446
Waddell M, Fallon HJ (1973) The effect of high-carbohydrate diets on liver triglyceride formation in the rat. J Clin Invest 52(11):2725–2731
Topping DL, Mayes PA (1972) The immediate effects of insulin and fructose on the metabolism of the perfused liver. Changes in lipoprotein secretion, fatty acid oxidation and esterification, lipogenesis and carbohydrate metabolism. Biochem J 126(2):295–311
Topping DL, Mayes PA (1976) Comparative effects of fructose and glucose on the lipid and carbohydrate metabolism of perfused rat liver. Br J Nutr 36(1):113–126
Sun SZ, Empie MW (2012) Fructose metabolism in humans. Nutr Metab 9(1):89. doi:10.1186/1743-7075-9-89
Tappy L, Le KA (2012) Does fructose consumption contribute to non-alcoholic fatty liver disease? Clin Res Hepatol Gastroenterol 36(6):554–560. doi:10.1016/j.clinre.2012.06.005
Rutledge AC, Adeli K (2007) Fructose and the metabolic syndrome: pathophysiology and molecular mechanisms. Nutr Rev 65(6 Pt 2):S13–S23
Sobrecases H, Lê KA, Bortolotti M, Schneiter P, Ith M, Kreis R, Boesch C, Tappy L (2010) Effects of short-term overfeeding with fructose, fat and fructose plus fat on plasma and hepatic lipids in healthy men. Diabetes Metab 36(3):244–246. doi:10.1016/j.diabet.2010.03.003
Lê KA, Ith M, Kreis R et al (2009) Fructose overconsumption causes dyslipidemia and ectopic lipid deposition in healthy subjects with and without a family history of type 2 diabetes. Am J Clin Nutr 89:1760–1765. doi:10.3945/ajcn.2008.27336
Coen PM, Goodpaster BH (2012) Role of intramyocellular lipids in human health. Trends Endocrinol Metab 23(8):391–398. doi:10.1016/j.tem.2012.05.009
Greco AV, Mingrone G, Giancaterini A, Manco M, Morroni M, Cinti S, Granzotto M, Vettor R, Camastra S, Ferrannini E (2002) Insulin resistance in morbid obesity. Diabetes 51(1):144–151. doi:10.2337/diabetes.51.1.144
McGarry JD (2002) Banting lecture 2001: dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes 51(1):7–18. doi:10.2337/diabetes.51.1.7
Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, Wynshaw-Boris A, Poli G, Olefsky J, Karin M (2005) IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med 11(2):191–198
Steinberg GR (2007) Inflammation in obesity is the common link between defects in fatty acid metabolism and insulin resistance. Cell Cycle 6(8):888–894. doi:10.4161/cc.6.8.4135
Spangenburg EE, Pratt SJ, Wohlers LM, Lovering RM (2011) Use of BODIPY (493/503) to visualize intramuscular lipid droplets in skeletal muscle. J Biomed Biotechnol 2011:598358. doi:10.1155/2011/598358
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72:248–254
Penna F, Costamagna D, Pin F, Camperi A, Fanzani A, Chiarpotto EM, Cavallini G, Bonelli G, Baccino FM, Costelli P (2013) Autophagic degradation contributes to muscle wasting in cancer cachexia. Am J Pathol 182(4):1367–1378. doi:10.1016/j.ajpath.2012.12.023
Bizeau ME, Pagliassotti MJ (2005) Hepatic adaptations to sucrose and fructose. Metab Clin Exp 54(9):1189–1201. doi:10.1016/j.metabol.2005.04.004
Samuel VT (2011) Fructose induced lipogenesis: from sugar to fat to insulin resistance. Trends Endocrinol Metab 22(2):60–65. doi:10.1016/j.tem.2010.10.003
Lass A, Zimmermann R, Oberer M, Zechner R (2011) Lipolysis—a highly regulated multi-enzyme complex mediates the catabolism of cellular fat stores. Prog Lipid Res 50(1):14–27. doi:10.1016/j.plipres.2010.10.004
Nunes PM, Wright AJ, Veltien A, van Asten JJ, Tack CJ, Jones JG, Heerschap A (2014) Dietary lipids do not contribute to the higher hepatic triglyceride levels of fructose-compared to glucose-fed mice. FASEB J 28(5):1988–1997. doi:10.1096/fj.13-241208
Christian P, Sacco J, Adeli K (2013) Autophagy: emerging roles in lipid homeostasis and metabolic control. Biochim Biophys Acta 1831(4):819–824. doi:10.1016/j.bbalip.2012.12.009
Unger RH (2003) Minireview: weapons of lean body mass destruction: the role of ectopic lipids in the metabolic syndrome. Endocrinology 144(12):5159–5165. doi:10.1210/en.2003-0870
Shortreed KE, Krause MP, Huang JH, Dhanani D, Moradi J, Ceddia RB, Hawke TJ (2009) Muscle-specific adaptations, impaired oxidative capacity and maintenance of contractile function characterize diet-induced obese mouse skeletal muscle. PLoS ONE 4(10):e7293. doi:10.1371/journal.pone.0007293
de Wilde J, Mohren R, van den Berg S, Boekschoten M, Dijk KW, de Groot P, Müller M, Mariman E, Smit E (2008) Short-term high fat-feeding results in morphological and metabolic adaptations in the skeletal muscle of C57BL/6J mice. Genomics 32(3):360–369. doi:10.1152/physiolgenomics.00219.2007
Gan Z, Rumsey J, Hazen BC, Lai L, Leone TC, Vega RB, Xie H, Conley KE, Auwerx J, Smith SR, Olson EN, Kralli A, Kelly DP (2013) Nuclear receptor/microRNA circuitry links muscle fiber type to energy metabolism. J Clin Invest 123(6):2564–2575. doi:10.1172/JCI67652
Bonnard C, Durand A, Peyrol S, Chanseaume E, Chauvin MA, Morio B, Vidal H, Rieusset J (2008) Mitochondrial dysfunction results from oxidative stress in the skeletal muscle of diet-induced insulin-resistant mice. J Clin Invest 118(2):789–800. doi:10.1172/JCI32601
Wu JJ, Quijano C, Chen E, Liu H, Cao L, Fergusson MM, Rovira II, Gutkind S, Daniels MP, Komatsu M, Finkel T (2009) Mitochondrial dysfunction and oxidative stress mediate the physiological impairment induced by the disruption of autophagy. Aging 1(4):425–437
Komatsu M, Waguri S, Ueno T, Iwata J, Murata S, Tanida I, Ezaki J, Mizushima N, Ohsumi Y, Uchiyama Y, Kominami E, Tanaka K, Chiba T (2005) Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J Cell Biol 169(3):425–434. doi:10.1083/jcb.200412022
Mortensen M, Ferguson DJ, Edelmann M, Kessler B, Morten KJ, Komatsu M, Simon AK (2010) Loss of autophagy in erythroid cells leads to defective removal of mitochondria and severe anemia in vivo. Proc Natl Acad Sci USA 107(2):832–837. doi:10.1073/pnas.0913170107
Masiero E, Agatea L, Mammucari C, Blaauw B, Loro E, Komatsu M, Metzger D, Reggiani C, Schiaffino S, Sandri M (2009) Autophagy is required to maintain muscle mass. Cell Metab 10(6):507–515. doi:10.1016/j.cmet.2009.10.008
Mellor KM, Bell JR, Young MJ, Ritchie RH, Delbridge LM (2011) Myocardial autophagy activation and suppressed survival signaling is associated with insulin resistance in fructose-fed mice. J Mol Cell Cardiol 50(6):1035–1043. doi:10.1016/j.yjmcc.2011.03.002
Kovsan J, Blüher M, Tarnovscki T, Klöting N, Kirshtein B, Madar L, Shai I, Golan R, Harman-Boehm I, Schön MR, Greenberg AS, Elazar Z, Bashan N, Rudich A (2011) Altered autophagy in human adipose tissues in obesity. J Clin Endocrinol Metab 96(2):E268–E277. doi:10.1210/jc.2010-1681
Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, Tanaka K, Cuervo AM, Czaja MJ (2009) Autophagy regulates lipid metabolism. Nature 458(7242):1131–1135. doi:10.1038/nature07976
Greenberg AS, Coleman RA, Kraemer FB, McManaman JL, Obin MS, Puri V, Yan QW, Miyoshi H, Mashek DG (2011) The role of lipid droplets in metabolic disease in rodents and humans. J Clin Invest 121(6):2102–2110. doi:10.1172/JCI46069
Cahová M, Daňková H, Páleníčková E, Papáčková Z, Kazdová L (2010) The autophagy-lysosomal pathway is involved in TAG degradation in the liver: the effect of high-sucrose and high-fat diet. Folia Biol 56(4):173–182
Komiya K, Uchida T, Ueno T, Koike M, Abe H, Hirose T, Kawamori R, Uchiyama Y, Kominami E, Fujitani Y, Watada H (2010) Free fatty acids stimulate autophagy in pancreatic β-cells via JNK pathway. Biochem Biophys Res Commun 401(4):561–567. doi:10.1016/j.bbrc.2010.09.101
Nowicki M, Serke H, Kosacka J, Müller K, Spanel-Borowski K (2010) Oxidized low-density lipoprotein (oxLDL) induces cell death in neuroblastoma and survival autophagy in schwannoma cells. Exp Mol Pathol 89(3):276–283. doi:10.1016/j.yexmp.2010.07.009
Pauloin A, Chat S, Péchoux C, Hue-Beauvais C, Droineau S, Galio L, Devinoy E, Chanat E (2010) Oleate and linoleate stimulate degradation of β-casein in prolactin-treated HC11 mouse mammary epithelial cells. Cell Tissue Res 340(1):91–102. doi:10.1007/s00441-009-0926-3
Khaldoun SA, Emond-Boisjoly MA, Chateau D, Carrière V, Lacasa M, Rousset M, Demignot S, Morel E (2014) Autophagosomes contribute to intracellular lipid distribution in enterocytes. Mol Biol Cell 25(1):118–132. doi:10.1091/mbc.E13-06-0324
Rodriguez J, Gilson H, Jamart C, Naslain D, Pierre N, Deldicque L, Francaux M (2014) Pomegranate and green tea extracts protect against ER stress induced by a high-fat diet in skeletal muscle of mice. Eur J Nutr 54(3):377–389. doi:10.1007/s00394-014-0717-9
Kashima J, Shintani-Ishida K, Nakajima M, Maeda H, Unuma K, Uchiyama Y, Yoshida K (2014) Immunohistochemical study of the autophagy marker microtubule-associated protein 1 light chain 3 in normal and steatotic human livers. Hepatol Res 44(7):779–787. doi:10.1111/hepr.12183
Wang H, Sun RQ, Zeng XY, Zhou X, Li S, Jo E, Molero JC, Ye JM (2015) Restoration of autophagy alleviates hepatic ER stress and impaired insulin signalling transduction in high fructose-fed male mice. Endocrinology 156(1):169–181. doi:10.1210/en.2014-1454
Li XZ, Sui CY, Chen Q, Chen XP, Zhang H, Zhou XP (2013) Promotion of autophagy at the maturation step by IL-6 is associated with the sustained mitogen-activated protein kinase/extracellular signal-regulated kinase activity. Mol Cell Biochem 380(1–2):219–227. doi:10.1007/s11010-013-1676-9
Johnston RD, Stephenson MC, Crossland H, Cordon SM, Palcidi E, Cox EF, Taylor MA, Aithal GP, Macdonald IA (2013) No difference between high-fructose and high-glucose diets on liver triacylglycerol or biochemistry in healthy overweight men. Gastroenterology 145(5):1016.e2–1025.e2. doi:10.1053/j.gastro.2013.07.012
Ngo Sock ET, Lê KA, Ith M, Kreis R, Boesch C, Tappy L (2010) Effects of a short-term overfeeding with fructose or glucose in healthy young males. Br J Nutr 103(7):939–943. doi:10.1017/S0007114509992819
Acknowledgments
This work was supported by Associazione Italiana per la Ricerca sul Cancro (AIRC, IG9153), Milano, Italy; University of Turin (ex-60 % funds), Italy; CRT Foundation (2010.1954), Italy.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors have not conflict of interest to declare.
Rights and permissions
About this article
Cite this article
De Stefanis, D., Mastrocola, R., Nigro, D. et al. Effects of chronic sugar consumption on lipid accumulation and autophagy in the skeletal muscle. Eur J Nutr 56, 363–373 (2017). https://doi.org/10.1007/s00394-015-1086-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-015-1086-8