Abstract
Purpose
Inflammatory bowel diseases (IBDs) are chronic inflammatory disorders with important impact on global health. Prebiotic and probiotic strategies are thought to be useful in the context of experimental IBD. Here, we compared the effects of preventive versus therapeutic treatment with a high fiber diet (prebiotic) in combination or not with Bifidobacterium longum (probiotic) in a murine model of chronic colitis.
Methods
Colitis was induced by adding dextran sulfate sodium (DSS) to drinking water for 6 days (acute colitis) or for 5 cycles of DSS (chronic colitis).
Results
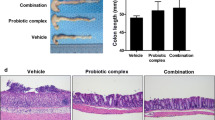
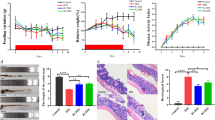
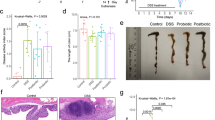
Administration of the high fiber diet protected from acute colitis. Protection was optimal when diet was started 20 days prior to DSS. A 5-day pretreatment with acetate, a short-chain fatty acid, provided partial protection against acute colitis. In chronic colitis, pretreatment with the high fiber diet attenuated clinical and inflammatory parameters of disease. However, when the treatment with the high fiber diet started after disease had been established, overall protection was minimal. Similarly, delayed treatment with acetate or B. longum did not provide any protection even when the probiotic was associated with the high fiber diet.
Conclusion
Preventive use of a high fiber diet or acetate clearly protects mice against acute and chronic damage induced by DSS in mice. However, protection is lost when therapies are initiated after disease has been established. These results suggest that any therapy aimed at modifying the gut environment (e.g., prebiotic or probiotic strategies) should be given early in the course of disease.





Similar content being viewed by others
References
Andoh A, Bamba T, Sasaki M (1999) Physiological and anti-inflammatory roles of dietary fiber and butyrate in intestinal functions. JPEN J Parenter Enteral Nutr 23:S70–S73
Ardizzone S, Bianchi Porro G (2005) Biologic therapy for inflammatory bowel disease. Drugs 65:2253–2286
Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, Rudensky AY (2013) Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504:451–455. doi:10.1038/nature12726
Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, Deng Y, Blennerhassett P, Macri J, McCoy KD, Verdu EF, Collins SM (2011) The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology 141:599–609, 609 e591–593. doi:10.1053/j.gastro.2011.04.052
Bercik P, Park AJ, Sinclair D, Khoshdel A, Lu J, Huang X, Deng Y, Blennerhassett PA, Fahnestock M, Moine D, Berger B, Huizinga JD, Kunze W, McLean PG, Bergonzelli GE, Collins SM, Verdu EF (2011) The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol Motil 23:1132–1139. doi:10.1111/j.1365-2982.2011.01796.x
Buanne P, Di Carlo E, Caputi L, Brandolini L, Mosca M, Cattani F, Pellegrini L, Biordi L, Coletti G, Sorrentino C, Fedele G, Colotta F, Melillo G, Bertini R (2007) Crucial pathophysiological role of CXCR2 in experimental ulcerative colitis in mice. J Leukoc Biol 82:1239–1246. doi:10.1189/jlb.0207118
Cryan JF, O’Mahony SM (2011) The microbiome-gut-brain axis: from bowel to behavior. Neurogastroenterol Motil 23:187–192. doi:10.1111/j.1365-2982.2010.01664.x
Cummings JH, Southgate DA, Branch WJ, Wiggins HS, Houston H, Jenkins DJ, Jivraj T, Hill MJ (1979) The digestion of pectin in the human gut and its effect on calcium absorption and large bowel function. Br J Nutr 41:477–485
De Cassan C, Fiorino G, Danese S (2012) Second-generation corticosteroids for the treatment of Crohn’s disease and ulcerative colitis: More effective and less side effects? Dig Dis 30:368–375. doi:10.1159/000338128
De Vadder F, Kovatcheva-Datchary P, Goncalves D, Vinera J, Zitoun C, Duchampt A, Backhed F, Mithieux G (2014) Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 156:84–96. doi:10.1016/j.cell.2013.12.016
Dongowski G, Lorenz A, Anger H (2000) Degradation of pectins with different degrees of esterification by Bacteroides thetaiotaomicron isolated from human gut flora. Appl Environ Microbiol 66:1321–1327
Duchmann R, Kaiser I, Hermann E, Mayet W, Ewe K, Meyer zum Buschenfelde KH (1995) Tolerance exists towards resident intestinal flora but is broken in active inflammatory bowel disease (IBD). Clin Exp Immunol 102:448–455
Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, Daly MJ, Steinhart AH, Abraham C, Regueiro M, Griffiths A, Dassopoulos T, Bitton A, Yang H, Targan S, Datta LW, Kistner EO, Schumm LP, Lee AT, Gregersen PK, Barmada MM, Rotter JI, Nicolae DL, Cho JH (2006) A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science 314:1461–1463. doi:10.1126/science.1135245
Foster JA, McVey Neufeld KA (2013) Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci 36:305–312. doi:10.1016/j.tins.2013.01.005
Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR (2007) Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA 104:13780–13785. doi:10.1073/pnas.0706625104
Galvez J, Rodriguez-Cabezas ME, Zarzuelo A (2005) Effects of dietary fiber on inflammatory bowel disease. Mol Nutr Food Res 49:601–608. doi:10.1002/mnfr.200500013
Geier MS, Butler RN, Giffard PM, Howarth GS (2007) Prebiotic and synbiotic fructooligosaccharide administration fails to reduce the severity of experimental colitis in rats. Dis Colon Rectum 50:1061–1069. doi:10.1007/s10350-007-0213-x
Hauser W, Janke KH, Klump B, Hinz A (2011) Anxiety and depression in patients with inflammatory bowel disease: comparisons with chronic liver disease patients and the general population. Inflamm Bowel Dis 17:621–632. doi:10.1002/ibd.21346
Hayes CL, Natividad JM, Jury J, Martin R, Langella P, Verdu EF (2014) Efficacy of Bifidobacterium breve NCC2950 against DSS-induced colitis is dependent on bacterial preparation and timing of administration. Benef Microbes 5:79–88. doi:10.3920/BM2013.0039
Heller F, Florian P, Bojarski C, Richter J, Christ M, Hillenbrand B, Mankertz J, Gitter AH, Burgel N, Fromm M, Zeitz M, Fuss I, Strober W, Schulzke JD (2005) Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology 129:550–564. doi:10.1016/j.gastro.2005.05.002
Kanauchi O, Suga T, Tochihara M, Hibi T, Naganuma M, Homma T, Asakura H, Nakano H, Takahama K, Fujiyama Y, Andoh A, Shimoyama T, Hida N, Haruma K, Koga H, Mitsuyama K, Sata M, Fukuda M, Kojima A, Bamba T (2002) Treatment of ulcerative colitis by feeding with germinated barley foodstuff: first report of a multicenter open control trial. J Gastroenterol 37(Suppl 14):67–72
Kirat D, Kondo K, Shimada R, Kato S (2009) Dietary pectin up-regulates monocaboxylate transporter 1 in the rat gastrointestinal tract. Exp Physiol 94:422–433. doi:10.1113/expphysiol.2009.046797
Komiyama Y, Mitsuyama K, Masuda J, Yamasaki H, Takedatsu H, Andoh A, Tsuruta O, Fukuda M, Kanauchi O (2011) Prebiotic treatment in experimental colitis reduces the risk of colitic cancer. J Gastroenterol Hepatol 26:1298–1308. doi:10.1111/j.1440-1746.2011.06690.x
Krieglstein CF, Anthoni C, Cerwinka WH, Stokes KY, Russell J, Grisham MB, Granger DN (2007) Role of blood- and tissue-associated inducible nitric-oxide synthase in colonic inflammation. Am J Pathol 170:490–496. doi:10.2353/ajpath.2007.060594
Kurtovic J, Segal I (2004) Recent advances in biological therapy for inflammatory bowel disease. Trop Gastroenterol 25:9–14
Loftus EV Jr (2004) Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology 126:1504–1517
Macfarlane S, Macfarlane GT (2003) Regulation of short-chain fatty acid production. Proc Nutr Soc 62:67–72. doi:10.1079/PNS2002207
Macia L, Tan J, Vieira AT, Leach K, Stanley D, Luong S, Maruya M, Ian McKenzie C, Hijikata A, Wong C, Binge L, Thorburn AN, Chevalier N, Ang C, Marino E, Robert R, Offermanns S, Teixeira MM, Moore RJ, Flavell RA, Fagarasan S, Mackay CR (2015) Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat Commun 6:6734. doi:10.1038/ncomms7734
Martinez-Medina M, Aldeguer X, Gonzalez-Huix F, Acero D, Garcia-Gil LJ (2006) Abnormal microbiota composition in the ileocolonic mucosa of Crohn’s disease patients as revealed by polymerase chain reaction-denaturing gradient gel electrophoresis. Inflamm Bowel Dis 12:1136–1145. doi:10.1097/01.mib.0000235828.09305.0c
Maslowski KM, Mackay CR (2011) Diet, gut microbiota and immune responses. Nat Immunol 12:5–9. doi:10.1038/ni0111-5
Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, Xavier RJ, Teixeira MM, Mackay CR (2009) Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 461:1282–1286. doi:10.1038/nature08530
Masui R, Sasaki M, Funaki Y, Ogasawara N, Mizuno M, Iida A, Izawa S, Kondo Y, Ito Y, Tamura Y, Yanamoto K, Noda H, Tanabe A, Okaniwa N, Yamaguchi Y, Iwamoto T, Kasugai K (2013) G protein-coupled receptor 43 moderates gut inflammation through cytokine regulation from mononuclear cells. Inflamm Bowel Dis 19:2848–2856. doi:10.1097/01.MIB.0000435444.14860.ea
Melgar S, Karlsson A, Michaelsson E (2005) Acute colitis induced by dextran sulfate sodium progresses to chronicity in C57BL/6 but not in BALB/c mice: correlation between symptoms and inflammation. Am J Physiol Gastrointest Liver Physiol 288:G1328–G1338. doi:10.1152/ajpgi.00467.2004
Miyauchi E, Ogita T, Miyamoto J, Kawamoto S, Morita H, Ohno H, Suzuki T, Tanabe S (2013) Bifidobacterium longum alleviates dextran sulfate sodium-induced colitis by suppressing IL-17A response: involvement of intestinal epithelial costimulatory molecules. PLoS ONE 8:e79735. doi:10.1371/journal.pone.0079735
Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, Kaplan GG (2012) Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 142:46–54 e42; quiz e30. doi:10.1053/j.gastro.2011.10.001
Moreau NM, Martin LJ, Toquet CS, Laboisse CL, Nguyen PG, Siliart BS, Dumon HJ, Champ MM (2003) Restoration of the integrity of rat caeco-colonic mucosa by resistant starch, but not by fructo-oligosaccharides, in dextran sulfate sodium-induced experimental colitis. Br J Nutr 90:75–85
Nagalingam NA, Kao JY, Young VB (2011) Microbial ecology of the murine gut associated with the development of dextran sodium sulfate-induced colitis. Inflamm Bowel Dis 17:917–926. doi:10.1002/ibd.21462
Ohkami H, Tazawa K, Yamashita I, Shimizu T, Murai K, Kobashi K, Fujimaki M (1995) Effects of apple pectin on fecal bacterial enzymes in azoxymethane-induced rat colon carcinogenesis. Jpn J Cancer Res 86:523–529
Reeves PG, Nielsen FH, Fahey GC Jr (1993) AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 123:1939–1951
Sakamoto N, Kono S, Wakai K, Fukuda Y, Satomi M, Shimoyama T, Inaba Y, Miyake Y, Sasaki S, Okamoto K, Kobashi G, Washio M, Yokoyama T, Date C, Tanaka H (2005) Dietary risk factors for inflammatory bowel disease: a multicenter case-control study in Japan. Inflamm Bowel Dis 11:154–163
Slyepchenko A, Carvalho AF, Cha DS, Kasper S, McIntyre RS (2014) Gut emotions—mechanisms of action of probiotics as novel therapeutic targets for depression and anxiety disorders. CNS Neurol Disord Drug Targets 13:1770–1786
Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly YM, Glickman JN, Garrett WS (2013) The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341:569–573. doi:10.1126/science.1241165
Souza DG, Soares AC, Pinho V, Torloni H, Reis LF, Teixeira MM, Dias AA (2002) Increased mortality and inflammation in tumor necrosis factor-stimulated gene-14 transgenic mice after ischemia and reperfusion injury. Am J Pathol 160:1755–1765
Souza TC, Silva AM, Drews JR, Gomes DA, Vinderola CG, Nicoli JR (2013) In vitro evaluation of Bifidobacterium strains of human origin for potential use in probiotic functional foods. Benef Microbes 4:179–186. doi:10.3920/BM2012.0052
Souza TC, Zacarias MF, Silva AM, Binetti A, Reinheimer J, Nicoli JR, Vinderola G (2012) Cell viability and immunostimulating and protective capacities of Bifidobacterium longum 51A are differentially affected by technological variables in fermented milks. J Appl Microbiol 112:1184–1192. doi:10.1111/j.1365-2672.2012.05280.x
Strath M, Warren DJ, Sanderson CJ (1985) Detection of eosinophils using an eosinophil peroxidase assay. Its use as an assay for eosinophil differentiation factors. J Immunol Methods 83:209–215
Vieira AT, Fagundes CT, Alessandri AL, Castor MG, Guabiraba R, Borges VO, Silveira KD, Vieira EL, Goncalves JL, Silva TA, Deruaz M, Proudfoot AE, Sousa LP, Teixeira MM (2009) Treatment with a novel chemokine-binding protein or eosinophil lineage-ablation protects mice from experimental colitis. Am J Pathol 175:2382–2391
Vieira AT, Macia L, Galvao I, Martins FS, Canesso MC, Amaral FA, Garcia CC, Maslowski KM, De Leon E, Shim D, Nicoli JR, Harper JL, Teixeira MM, Mackay CR (2015) A role for gut microbiota and the metabolite-sensing receptor GPR43 in a murine model of gout. Arthritis Rheumatol 67:1646–1656. doi:10.1002/art.39107
Zhao HM, Huang XY, Zuo ZQ, Pan QH, Ao MY, Zhou F, Liu HN, Liu ZY, Liu DY (2013) Probiotics increase T regulatory cells and reduce severity of experimental colitis in mice. World J Gastroenterol 19:742–749. doi:10.3748/wjg.v19.i5.742
Acknowledgments
This work received financial support from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil), Fundação de Amparo e Pesquisas do Estado Minas Gerais (FAPEMIG, Brazil), National Institute of Science of Technology in Dengue (INCT in Dengue, CNPq, Brazil). We thank Frankcinéia Assis and Ilma Marçal for technical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Silveira, A.L.M., Ferreira, A.V.M., de Oliveira, M.C. et al. Preventive rather than therapeutic treatment with high fiber diet attenuates clinical and inflammatory markers of acute and chronic DSS-induced colitis in mice. Eur J Nutr 56, 179–191 (2017). https://doi.org/10.1007/s00394-015-1068-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-015-1068-x