Abstract
Purpose
Using a diet-induced obesity (DIO) mouse model, we investigated the antidiabetic effect of Labrador tea [Rhododendron groenlandicum (Oeder) Kron and Judd], a beverage and medicinal tea used by the Cree Nations of northern Quebec.
Methods
C57BL6 mice were divided into five groups and given standard chow (~4 % of lipids) or high-fat diet (~35 % of lipids) for 8 weeks until they became obese and insulin resistant. Treatment began by adding the plant extract at three doses (125, 250 and 500 mg/kg) to the high-fat diet for another 8 weeks. At the end of the study, insulin-sensitive tissues (liver, skeletal muscle, adipose tissue) were collected to investigate the plant’s molecular mechanisms.
Results
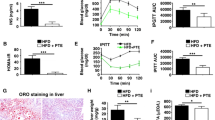
Labrador tea significantly reduced blood glucose (13 %), the response to an oral glucose tolerance test (18.2 %) and plasma insulin (65 %) while preventing hepatic steatosis (42 % reduction in hepatic triglyceride levels) in DIO mice. It stimulated insulin-dependent Akt pathway (55 %) and increased the expression of GLUT4 (53 %) in skeletal muscle. In the liver, Labrador tea stimulated the insulin-dependent Akt and the insulin-independent AMP-activated protein kinase pathways. The improvement in hepatic steatosis observed in DIO-treated mice was associated with a reduction in inflammation (through the IKK α/β) and a decrease in the hepatic content of SREBP-1 (39 %).
Conclusions
Labrador tea exerts potential antidiabetic action by improving insulin sensitivity and mitigating high-fat diet-induced obesity and hyperglycemia. They validate the safety and efficacy of this plant, a promising candidate for culturally relevant complementary treatment in Cree diabetics.







Similar content being viewed by others
References
Hegele RA (2001) Genes and environment in type 2 diabetes and atherosclerosis in aboriginal Canadians. Curr Atheroscler Rep 3:216–221
Brassard P, Robinson E, Lavallee C (1993) Prevalence of diabetes mellitus among the James Bay Cree of northern Quebec. Can Med Assoc J 149:303–307
Leduc C, Coonishish J, Haddad P, Cuerrier A (2006) Plants used by the Cree Nation of Eeyou Istchee (Quebec, Canada) for the treatment of diabetes: a novel approach in quantitative ethnobotany. J Ethnopharmacol 105:55–63
Hedrick UP (1972) Sturtevant’s edible plants of the world. Dover, New York
Saleem A, Harris CS, Asim M et al (2010) A RP-HPLC–DAD-APCI/MSD method for the characterisation of medicinal Ericaceae used by the Eeyou Istchee Cree First Nations. Phytochem Anal 21:328–339
Rapinski M, Liu R, Saleem A, Arnason JT, Cuerrier A (2014) Environmental trends in the variation of biologically active phenolic compounds in Labrador tea, Rhododendron groenlandicum, from northern Quebec, Canada. Botany 92:783–794
Spoor DC, Martineau LC, Leduc C et al (2006) Selected plant species from the Cree pharmacopoeia of northern Quebec possess anti-diabetic potential. Can J Physiol Pharmacol 84:847–858
Martineau LC, Adeyiwola-Spoor DC, Vallerand D, Afshar A, Arnason JT, Haddad PS (2010) Enhancement of muscle cell glucose uptake by medicinal plant species of Canada’s native populations is mediated by a common, metformin-like mechanism. J Ethnopharmacol 127:396–406
Jiang T, Wang Z, Proctor G et al (2005) Diet-induced obesity in C57BL/6J mice causes increased renal lipid accumulation and glomerulosclerosis via a sterol regulatory element-binding protein-1c-dependent pathway. J Biol Chem 280:32317–32325
Bray GA, Lovejoy JC, Smith SR et al (2002) The influence of different fats and fatty acids on obesity, insulin resistance and inflammation. J Nutr 132:2488–2491
Wallberg-Henriksson H, Zierath JR (2001) GLUT4: a key player regulating glucose homeostasis? Insights from transgenic and knockout mice (review). Mol Membr Biol 18:205–211
Saltiel AR, Kahn CR (2001) Insulin signalling and the regulation of glucose and lipid metabolism. Nature 414:799–806
Gibbs EM, Stock JL, McCoid SC et al (1995) Glycemic improvement in diabetic db/db mice by overexpression of the human insulin-regulatable glucose transporter (GLUT4). J Clin Investig 95:1512–1518
Kurth-Kraczek EJ, Hirshman MF, Goodyear LJ, Winder WW (1999) 5′ AMP-activated protein kinase activation causes GLUT4 translocation in skeletal muscle. Diabetes 48:1667–1671
Farmer SR (2005) Regulation of PPARgamma activity during adipogenesis. Int J Obes 29(Suppl 1):S13–S16
Rasouli N, Kern PA (2008) Adipocytokines and the metabolic complications of obesity. J Clin Endocrinol Metab 93:S64–S73
Diez JJ, Iglesias P (2003) The role of the novel adipocyte-derived hormone adiponectin in human disease. Eur J Endocrinol 148:293–300
Ukkola O, Santaniemi M (2002) Adiponectin: a link between excess adiposity and associated comorbidities? J Mol Med 80:696–702
Considine RV, Sinha MK, Heiman ML et al (1996) Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med 334:292–295
Shimano H (2001) Sterol regulatory element-binding proteins (SREBPs): transcriptional regulators of lipid synthetic genes. Prog Lipid Res 40:439–452
Tobe K, Suzuki R, Aoyama M et al (2001) Increased expression of the sterol regulatory element-binding protein-1 gene in insulin receptor substrate-2(−/−) mouse liver. J Biol Chem 276:38337–38340
Postic C, Dentin R, Girard J (2004) Role of the liver in the control of carbohydrate and lipid homeostasis. Diabetes Metab 30:398–408
Marchesini G, Brizi M, Bianchi G et al (2001) Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes 50:1844–1850
Arkan MC, Hevener AL, Greten FR et al (2005) IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med 11:191–198
Reagan-Shaw S, Nihal M, Ahmad N (2008) Dose translation from animal to human studies revisited. FASEB J 22:659–661
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419
Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226:497–509
Benhaddou-Andaloussi A, Martineau LC, Vallerand D et al (2010) Multiple molecular targets underlie the antidiabetic effect of Nigella sativa seed extract in skeletal muscle, adipocyte and liver cells. Diabetes Obes Metab 12:148–157
Surwit RS, Kuhn CM, Cochrane C, McCubbin JA, Feinglos MN (1988) Diet-induced type II diabetes in C57BL/6J mice. Diabetes 37:1163–1167
Unnikrishnan MK, Veerapur V, Nayak Y, Mudgal PP, Mathew G (2013) Antidiabetic antihyperlipidemic and antioxidant effects of the flavonoids. In: Watson VRP, Zibadi S, Ronald R (eds) Polyphenols in human health and disease, 1st edn. Elsevier Inc., USA, pp 143–161
Takahashi M, Miyashita M, Suzuki K et al (2014) Acute ingestion of catechin-rich green tea improves postprandial glucose status and increases serum thioredoxin concentrations in postmenopausal women. Br J Nutr 112:1542–1550
Curtis PJ, Sampson M, Potter J, Dhatariya K, Kroon PA, Cassidy A (2012) Chronic ingestion of flavan-3-ols and isoflavones improves insulin sensitivity and lipoprotein status and attenuates estimated 10-year CVD risk in medicated postmenopausal women with type 2 diabetes: a 1-year, double-blind, randomized, controlled trial. Diabetes Care 35:226–232
Xie Y, Xie Z (2015) Experimental models of high fat obesity and leucine supplementation. In: Rajendram R, Preedy VR, Patel VB, Chain Branched (eds) Amino Acids in Clinical Nutrition. Springer, New York, pp 219–227
Karlsson HK, Zierath JR, Kane S, Krook A, Lienhard GE, Wallberg-Henriksson H (2005) Insulin-stimulated phosphorylation of the Akt substrate AS160 is impaired in skeletal muscle of type 2 diabetic subjects. Diabetes 54:1692–1697
Sanz P (2008) AMP-activated protein kinase: structure and regulation. Curr Protein Pept Sci 9:478–492
Haber BA, Chin S, Chuang E, Buikhuisen W, Naji A, Taub R (1995) High levels of glucose-6-phosphatase gene and protein expression reflect an adaptive response in proliferating liver and diabetes. J Clin Investig 95:832–841
Clore JN, Stillman J, Sugerman H (2000) Glucose-6-phosphatase flux in vitro is increased in type 2 diabetes. Diabetes 49:969–974
Nachar A, Vallerand D, Musallam L et al (2013) The action of antidiabetic plants of the Canadian James bay Cree traditional pharmacopeia on key enzymes of hepatic glucose homeostasis. Evidence-based Complement Altern Med 2013:189819
Samuel VT, Liu ZX, Qu X et al (2004) Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J Biol Chem 279:32345–32353
Foretz M, Taleux N, Guigas B et al (2006) Regulation of energy metabolism by AMPK: a novel therapeutic approach for the treatment of metabolic and cardiovascular diseases. Med Sci M/S 22:381–388
Hardie DG, Scott JW, Pan DA, Hudson ER (2003) Management of cellular energy by the AMP-activated protein kinase system. FEBS Lett 546:113–120
Winder WW, Hardie DG (1999) AMP-activated protein kinase, a metabolic master switch: possible roles in type 2 diabetes. Am J Physiol 277:E1–E10
Fatehi-Hassanabad Z, Chan CB (2005) Transcriptional regulation of lipid metabolism by fatty acids: a key determinant of pancreatic beta-cell function. Nutr Metab 2:1
Poynter ME, Daynes RA (1998) Peroxisome proliferator-activated receptor alpha activation modulates cellular redox status, represses nuclear factor-kappaB signaling, and reduces inflammatory cytokine production in aging. J Biol Chem 273:32833–32841
Kohjima M, Higuchi N, Kato M et al (2008) SREBP-1c, regulated by the insulin and AMPK signaling pathways, plays a role in nonalcoholic fatty liver disease. Int J Mol Med 21:507–511
Yahagi N, Shimano H, Hasty AH et al (2002) Absence of sterol regulatory element-binding protein-1 (SREBP-1) ameliorates fatty livers but not obesity or insulin resistance in Lep(ob)/Lep(ob) mice. J Biol Chem 277:19353–19357
Lowell BB (1999) PPARgamma: an essential regulator of adipogenesis and modulator of fat cell function. Cell 99:239–242
de Souza CJ, Eckhardt M, Gagen K et al (2001) Effects of pioglitazone on adipose tissue remodeling within the setting of obesity and insulin resistance. Diabetes 50:1863–1871
Schilling TM, Kolsch M, Larra MF et al (2013) For whom the bell (curve) tolls: cortisol rapidly affects memory retrieval by an inverted U-shaped dose–response relationship. Psychoneuroendocrinology 38:1565–1572
Acknowledgments
Very special thanks are due to E. Coon Come, M. Gunner, C. Husky Swallow, J. Husky Swallow, R. Loon and G. Loon from the Cree Nation of Mistissini as well as 27 other elders and healers, who kindly agreed to be interviewed. They made this article possible by allowing us to use, for the purposes of this research, their knowledge relating to medicinal plants, transmitted to them by their elders. Their trust has also enabled a useful exchange between indigenous knowledge and Western science. This work was supported by a Team Grant from the Canadian Institutes of Health Research (CIHR Team in Aboriginal Antidiabetic Medicines) to P.S.H. and J.T.A. It was conducted with the consent and support of the Cree Nation of Mistissini, of the Whapmagoostui First Nation, of the Cree Nation of Nemaska, of the Waskaganish First Nation and of the Cree Board of Health and Social Services of James Bay (Quebec, Canada).
Conflict of interest
Authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Meriem Ouchfoun and Hoda M. Eid have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Ouchfoun, M., Eid, H.M., Musallam, L. et al. Labrador tea (Rhododendron groenlandicum) attenuates insulin resistance in a diet-induced obesity mouse model. Eur J Nutr 55, 941–954 (2016). https://doi.org/10.1007/s00394-015-0908-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-015-0908-z