Abstract
Purpose
Evidence suggests that soy foods have chemoprotective properties that may reduce the risk of certain cancers such as breast and prostate cancer. However, data involving gastrointestinal (GI) have been limited, and the evidence remains controversial. This study aims to determine the potential relationship between dietary soy intake and GI cancer risk with an evaluation of the effects of isoflavone as an active soy constituent.
Methods
Relevant studies were identified after literature search via electronic databases through May 2014. Subgroup analysis for isoflavone intake (studies n = 10) was performed. Covariants including gender types, anatomical subsites and preparation methods were also evaluated. Pooled adjusted odds ratios (ORs) comparing highest and lowest categories of dietary pattern scores were calculated using a random effects model.
Results
Twenty-two case–control and 18 cohort studies were included for meta-analysis, which contained a total of 633,476 participants and 13,639 GI cancer cases. The combined OR was calculated as 0.93 (95 % CI 0.87–0.99; p value heterogeneity = 0.01), showing only a slight decrease in risk, the association was stronger for colon cancer (OR 0.92; 95 % CI 0.96–0.99; p value heterogeneity = 0.163) and colorectal cancer (CRC) (OR 0.92; 95 % CI 0.87–0.97; p value heterogeneity = 0.3). Subgroup analysis for isoflavone intake showed a statistically significant risk reduction with a risk estimate of 0.73 (95 % CI 0.59–0.92; p value heterogeneity = 0), and particularly for CRC (OR 0.76; 95 % CI 0.59–0.98; p value heterogeneity = 0).
Conclusion
This study provides evidence that soy intake as a food group is only associated with a small reduction in GI cancer risk. Separate analysis for dietary isoflavone intakes suggests a stronger inverse association.
Similar content being viewed by others
Introduction
Diet plays an important role in the etiology of certain cancers; however, establishing precisely which dietary factors account for this influence has proved to be difficult. Certain food groups such as vegetables and fruits contain phytochemicals with anti-carcinogenic properties. Soy foods and soybean components, in recent years, have particularly received considerable attention for their potential role in reducing cancer risk. Although the breast and prostate cancer has been the focus of most interest, there is an expanding body of literature on the possible association between soy foods and gastrointestinal (GI) cancers.
A number of anti-carcinogenic phytochemicals in soybeans have been identified, including phytosterols, phenolic acids and protease inhibitors [1]. Among these, most attention has been focused on the component isoflavones. Isoflavones have a limited distribution in nature with soybeans being its main source [2]. Although many investigators have focused on its anti-estrogenic properties and its potential for preventing hormonally mediated cancers, they also act as antioxidants and possess other anti-carcinogenic activities, including inhibition of angiogenesis [3], topoisomerase [4] and tyrosine kinase [5].
Genistein, one of the predominant isoflavones in soy, has been demonstrated to inhibit proliferation of cultured cells, including normal and transformed intestinal epithelial cells in a number of rodent and human cancer cell lines [6].
While the mechanisms that underpin an inverse relation between soy consumption and GI cancer risk seem plausible, the evidence from epidemiological data to date has been inconsistent and controversial. A recent meta-analysis examining four cohort and nine case–control studies on soy consumption and colorectal cancer (CRC) risk found a 21 % risk reduction in women; however, the overall analysis showed no significant association [7]. Two separate meta-analyses reviewed the effects of soy foods on gastric cancer risk on different population groups [1, 8]. Pooled results from their meta-analyses suggested that the risk of stomach cancer may depend on whether the soy food was fermented or non-fermented, with non-fermented products being associated with a greater risk reduction. Results from in vitro and in vivo animal studies have also been inconsistent with regard to a protective effect of soy and colorectal cancers. Five studies showed a statistically significant effect of soy diet or isoflavone supplement inhibiting the formation of aberrant crypt foci in rats, which are preneoplastic lesions of CRC [9]. Three studies that tested soy protein isolate on chemically induced colon cancer did not note a reduction in carcinogenesis [10–12].
Previous epidemiological studies have used different parameters in reporting the serving sizes and have included different types of soy in their analysis, making interpretation of results difficult. Gender influence has also been an area of interest given the anti-estrogenic properties of soy isoflavone. This systematic review and meta-analysis aim to summarize epidemiological findings to explore and clarify the relationship between soy intake and GI cancer, including subgroup analyses of dietary isoflavone intake, anatomical subsites, the influence of gender and types of soy food consumed.
Methods
Study protocol
Literature searches of epidemiological studies in this systematic review were performed using the meta-analysis of observational studies in epidemiology (MOOSE) guidelines where possible [13]. The following electronic databases were searched: MEDLINE, PubMed, ISI Web of Science, Current Contents Connect and Embase. The search included all studies published up to May 27, 2014. Key terms including ‘Soy’ OR ‘Isoflavone’ AND ‘Gastrointestinal neoplasm’ were searched as text words and as exploded medical subject headings where possible. References in the relevant review articles from the bibliographic database search were also checked for appropriate studies. No language restrictions were used in either the search or study selection. A search for unpublished literature was not performed.
Study selection
The following inclusion criteria were applied in the screening of articles: (1) original data on soy consumption and GI neoplasms risk, that of the esophagus, stomach and/or colorectum, were provided; (2) the risk point estimate was reported as OR or RR, or the data were presented such that an OR could be calculated; (3) the 95 % confidence interval (CI) was reported, or the data were presented such that the CI could be calculated.
Data extraction
Data extraction was performed via a standardized data extraction form, collecting information on the publication year, study design, number of cases and controls, total sample size, temporal direction, population type, country, ethnicity of sample group, case–control matching, mean age, response rate, exposure, neoplasm type, number of adjusted variables, method of intake measurement, and the risk estimates or data used to calculate the risk estimates, CIs. Quality of the studies was not assessed, and authors were not contacted for missing data. Adjusted odds ratios (ORs) were extracted in preference to non-adjusted odds ratios; however, where OR was not provided, unadjusted ORs and CIs were calculated. Where more than one adjusted ratio was reported, the ratio with the highest number of adjusted variables was chosen. Where multiple risk estimates were available in the same study, for example, due to the use of mutually exclusive comparator groups, they were included as separate risk estimates. Where ORs were provided in tertiles, quartiles or quintiles, the middle tiles were included.
Statistical analysis
Pooled OR and 95 % confidence intervals were calculated for the effect of soy intake on the risk of GI neoplasms using a random effects model, model of DerSimmonian and Laird [14]. Heterogeneity with Cochran’s Q statistic was tested, with p < 0.10 indicating heterogeneity, and the degree of heterogeneity was quantified using the I 2 statistic, which represents the percentage of the total variability across studies due to heterogeneity. I 2 values of 25, 50 and 75 % corresponded to low, moderate and high degrees of heterogeneity, respectively [15]. Publication bias was quantified using the Egger’s regression model [16]. All analyses were performed with comprehensive meta-analysis (version 2.0).
Effect modification was tested between soy intake and other covariates (country, gender, GI site, specific soy foods and method of soy preparation) through the addition of multiplicative interaction terms into the model. A separate analysis for dietary isoflavone intake and GI cancer risk was performed. As different methods were used to report soy intake, sensitivity analysis on serving sizes involved categorizing studies using a standardized measurement-consumption of three or more times per week and excluding those that did not specify intake frequency. Analyses were performed comparing studies that used validated versus non-validated FFQs.
Results
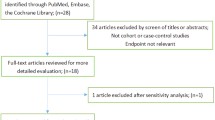
The literature searches identified 348 articles for evaluation. Title and abstract screening excluded 292 articles due to duplicates, non-human or non-original research. Full text screening excluded 15 articles—seven studies did not include soy or isoflavone in the dietary assessment, confidence intervals could not be calculated in six studies, and two studies did not present original data. Twenty-two case–control and 18 cohort studies were included in the final meta-analysis, which contained a total of 633,476 participants and 13,639 GI cancer cases. Characteristics of studies are outlined in Table 1.
Eighteen case–controls and 16 cohort studies provided data on soy intake. The combined OR was consistent with an inverse association between soy consumption and GI cancer, calculated to be 0.93 (95 % CI 0.87–0.99; p value heterogeneity = 0.01), showing only a slight decrease in risk. Results for both case–control (OR 0.86; 95 % CI 0.72–1.02); p value heterogeneity = 0.07) and cohort studies (OR 0.97; 95 % CI 0.90–1.03; p value heterogeneity = 0.21) were similar. Analysis of variables including country, gender, study design, type of soy consumed and cancer type was conducted (Table 2). Egger’s regression analysis for assessment of publication bias of the studies included in this meta-analysis found no significant bias (p < 0.001) (Fig. 1).
When analyzing according to GI sites, colon cancer (OR 0.92; 95 % CI 0.96–0.99; p value heterogeneity = 0.163) and CRC (OR 0.92; 95 % CI 0.87–0.97; p value heterogeneity = 0.3) showed a weak but statistically significant reduction in risk. Gastric cancer and rectal cancer were associated with a small reduction in risk as well; however, the results were not significant. Only two studies examined the effects of soy intake on esophageal cancer risk and hence were not included in the analysis.
Seven case–control and 12 cohort studies specifically examined gender variation on the influence of soy intake on GI cancer risk. Our analysis showed little difference between men and women. There is a modest but statistically significant inverse association for both men (OR 0.93; 95 % CI 0.87–0.99; p value heterogeneity = 0.01) and women (OR 0.92; 95 % CI 0.85–0.99; p value heterogeneity = 0.03). Subgroup site-specific analysis showed that the association was statistically significant for colon cancer in women (OR 0.85; 95 % CI 0.73–0.99; p value heterogeneity = 0.4).
Of the 41 included studies, only seven were from Western countries and the rest were of Asian origin. Studies from China and Japan showed a reduced risk of GI cancers with ORs of 0.81 (95 % CI 0.74–0.87; p value heterogeneity < 0.001) and 0.95 (95 % CI 0.92–0.99; p value heterogeneity = 0.03), respectively.
Results for fermented and non-fermented soy products were similar, yielding ORs of 0.97 (95 % CI 0.88–1.06; p value heterogeneity = 49.13) and 1.03 (95 % CI 0.92–1.15; p value heterogeneity = 0.104), respectively. Due to a large number of studies from Asian countries assessing tofu (bean curd) and miso (soybean paste soup) intake, we further conducted stratified analysis to determine the effects of specific soy products. Our results showed no significant association or difference between intake of either soy product and GI cancer risk.
Five case–control and five cohort studies included the subgroup analysis on dietary isoflavone intake (Fig. 2). Combined analysis yielded a statistically significant inverse association with a risk estimate of 0.73 (95 % CI 0.59–0.92; p value heterogeneity = 0). Site-specific evaluation showed a significant inverse association with CRC (OR 0.76; 95 % CI 0.59–0.98; p value heterogeneity = 0) only. Only five studies provided separate data on men and women; our analysis yielded very similar results for gender.
Of the studies included in our analysis, only five case–control and seven cohort studies employed a validated food frequency questionnaire (FFQ) to measure soy intake. Sensitivity analysis that excluded non-validated FFQs reported a risk estimate of 0.95 (95 % CI 0.882–1.01; p value heterogeneity = 0.004), yielding similar results for when all studies were included.
Discussion
Our results show that dietary isoflavone intake is strongly associated with a decrease in risk of developing GI cancers. Evidence for the consumption of soy and GI cancer intake has not been as consistent, showing only a small decrease in risk. To our knowledge, this is the first systematic review to investigate soy and dietary isoflavone intake in relation to GI cancer risk, taking into account the outcome of different anatomical subsites, and in influence of gender differences and other covariants.
All but one study included in our analysis of the effects isoflavone intake have been published within the past decade. Results from seven out of the ten studies support the hypothesis that isoflavone is inversely associated with GI cancer risk. Soybeans and soy products contain roughly 1–3 mg isoflavones per gram protein. Isoflavones are also known as ‘phytoestrogens’ because of the structural and metabolic similarities to mammalian estrogens [17]. They help to regulate estrogen levels in the body by acting on estrogen receptors in the body. Epidemiological and clinical studies have shown a significant decrease in CRC risk among postmenopausal women who used hormone replacement therapies, providing evidence that CRC is hormone sensitive [18]. In human colorectal tumor cells, estrogen receptor gene expression was diminished or absent; introduction of an exogenous estrogen receptor gene in cultured colon carcinoma cells resulted in marked growth suppression [19]. These studies suggest that endogenous ovarian hormones and even phytoestrogens might modulate CRC risk. Isoflavones also have other chemoprotective properties. One small-scale intervention study showed that soy protein containing isoflavone reduces crypt cell proliferation in colon mucosa biopsies from male and female subjects with a history of colon polyps or colon cancer [20]. Other anticancer activities included a decrease in abnormal cellular proliferation and the induction of apoptosis and inhibition angiogenesis [3, 5]. When examining site-specific differences, currently there are no hypothesized mechanisms for soy contributing to the development of gastric or rectal cancer, but it could be postulated that mechanisms proposed for colon cancer are similar.
Our analysis revealed only a very small decrease in GI cancer risk. This discrepancy may relate to several modifying factors, including the specific type of soy consumed, its preparation and gender differences. Wu et al. [8] recently did a thorough review and a meta-analysis of soy foods and the risk of stomach cancer. Pooled results from the meta-analysis suggested that the risk of stomach cancer may depend on the method of preparation of the soy food. The findings indicated that the risk was lower in association with high intake of non-fermented soy foods and higher with high consumption of fermented soy foods. Messina et al.’s review of in vitro and in vivo data on colon cancer provided similar suggestions [1]. Results from a recent meta-analysis on soy intake and CRC risk, which reviewed 15 case–control and cohort studies, generally suggest an inverse association between higher soy consumption and colon cancer onset; however, nearly all of the confidence intervals overlap 1.0 [7]. Of the six case–control studies that evaluated the association between soy consumption and rectal cancer (732 cases), the point estimates generally suggest an inverse association with unfermented soy consumption and rectal cancer onset but not fermented soy products. The same study also reported a 21 % reduction in CRC risk in women. These findings were not seen in our stratified analyses.
Results from human trials and animal studies have also been conflicting. One human intervention trial providing either a soy or casein supplement for 12 months reported reductions in colon epithelial cell proliferation in the soy group [20]. Another showed that miso dose-dependently lowered the mean numbers of aberrant crypt foci, an early marker for tumorigenesis, in the colons of male rats [21]. On the other hand, of the three studies that specifically examined the effect of soy protein isolate on chemically induced colonic cancer, none found a reduction in the rate of carcinogenesis [10–12]. Furthermore, one study found that soy protein isolate increased colonic cell proliferation [22].
The ability of these studies to detect a true relationship depends on a number of aspects of the assessment of soy food consumption. Results may be influenced by inconsistent measurement tools for evaluating dietary intake, sensitivity of the questionnaire or interview to assess relevant food items for soy intake, and/or the soy content of food. Few studies were originally designed to test the effect of soy as a risk factor or included portion sizes. Measurement standards differed across the studies; the categories of high consumption ranged from a low of one time or more per week to a high of three times or more per day. This inconsistency and the absence of standardized and comprehensive measurements undermine existing evidence. To address this issue, we categorized all relevant studies using standardized measurements of consumption frequency in our analysis to provide a more unified body of evidence.
Potential misclassification can also arise from the difference in composition of soy in the Asian and Western diet that can complicate interpretation of the findings. Asian studies, which comprise the majority of our data, generally measured intake of only one or two types of soy, such as miso and tofu, whereas the soy type was not specified in most studies from Western countries. This leads to potential misclassification and can complicate interpretation of the findings. Results from our stratified analysis did not show any significant difference between studies that examined soy as a whole food group versus specific soy types and method of preparation, suggesting that such modifying factors do not play a prominent role. However, on examining the differences between countries, the correlation of the protective effects of soy with GI cancer was stronger in countries such as China and Japan, as compared with USA. This may relate to higher levels of soy intake and a greater variety on the type of soy consumed.
In addition, as with all questionnaire-based retrospective studies, reliability of results might suffer from measurement and reporting bias. Validation is important to assess the degree to which the questionnaire measures items for which it has been designed. Incorrect information may over or underestimate associations between dietary factors and diseases. Global questions on soy consumption rather underestimate actual intake, whereas a higher number of questions on differently prepared soy foods result in more accurate levels of intake. The observed risk estimate for case–control studies was similar to that for cohort studies, which suggests that in this instance recall bias does not have a prominent effect. Our sensitivity analysis also revealed similar results when non-validated studies were excluded.
Moreover, while most studies adjusted for age and gender in the calculation of risk estimates, not all parameters were considered. Many studies did not appropriately adjust for total energy intake. Total energy intake is mainly a consequence of variations in body size, physical activity and metabolic efficiency. Failure to adjust for such factors can obscure associations between nutrient intakes and disease risk. Intakes of certain nutrients that are correlated with total energy intake may have a non-causal association with disease as a result of confounding by total energy intake. While a meta-analysis would not adequately adjust for this, this is less of a problem for nutrients such as isoflavone that contribute only a small amount of total energy [63].
In summary, the main findings of this study support an inverse association between isoflavone intake and GI cancer risk. Evidence for the chemoprotective effects of soy as a food group in general is much weaker, with only a small decrease in GI cancer risk. The correlation appears to be significant in CRC and among Asian populations. Further research should evaluate isoflavone content within different soy types when measuring exposure, paying attention to the patterns of consumption among different ethnic groups and adjusting for cofounders. Measurements of consumption should be provided in quantifiable terms, using FFQs validated against multiple days of dietary records and adjusting for total energy intake.
References
Messina M, Bennink M (1998) Soyfoods, isoflavones and risk of colonic cancer: a review of the in vitro and in vivo data. Baillieres Clin Endocrinol Metab 12:707–728
Wang H, Murphy PA (1994) Isoflavone content in commercial soybean foods. J Afric Food Chem 42:1666–1673
Fotsis T, Pepper M, Adlercreutz H, Hase T, Montesano R, Schweigerer L (1995) Genistein, a dietary ingested isoflavonoid, inhibits cell proliferation and in vitro angiogenesis. J Nutr 125:790S–797S
Markovits J, Linassier C, Fosse P, Couprie J, Pierre J, Jacquemin-Sablon A, Saucier JM, Le Pecq JB, Larsen AK (1989) Inhibitory effects of the tyrosine kinase inhibitor genistein on mammalian DNA topoisomerase II. Cancer Res 49:5111–5117
Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, Shiyba M, Fukami Y (1987) Genistein, a specific inhibitor of tyrosine-specific kinase. J Biol Chem 25:5592–5595
Booth C, Hargreaves DF, Hadfield JA, McGown AT, Potten CS (1999) Isoflavones inhibit intestinal epithelial cell proliferation and induce apoptosis in vitro. Br J Cancer 80:1550–1557
Yan L, Spitznagel EL, Bosland MC (2010) Soy consumption and colorectal cancer risk in humans: a meta-analysis. Cancer Epidemiol Biomarkers Prev 19:148–158
Wu AH, Yang D, Pike MC (2000) A meta-analysis of soyfoods and risk of stomach cancer: the problem of potential confounders. Cancer Epidemiol Biomarkers Prev 9:1051–1058
Lechner D, Kallay E, Cross HS (2005) Phytoestrogens and colorectal cancer prevention. Vitam Horm 70:169–198
Clinton S, Destree R, Anderson D (1979) 1,2-Dimethylhydrazube induced intestinal cancer in rats fed beef or soy protein. Nutr Rep Int 20:335–342
Reddy B, Narisawa T, Weisburger J (1976) Effect of a diet with high levels of protein and fat on colon carcinogenesis in F344 rats treated with 1,2-Dimethylhydrazine. J Nat Cancer Inst 57:1559–1566
Monsma D, Eghtedary K, Klurfeld D (1997) Colon cancer and aberrant crpy formation in ras fed different types of meat. FASEB J 11:A576
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA 283:2008–2012
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634
Zhou Y, Lee AS (1998) Mechanism for the suppression of the mammalian stress response by genistein, an anticancer phytoestrogen from soy. J Nat Cancer Inst 90:381–388
Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J, Writing Group for the Women’s Health Initiative I (2002) Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the women’s health initiative randomized controlled trial. JAMA 288:321–333
Issa JP, Ottaviano YL, Celano P, Hamilton SR, Davidson NE, Baylin SB (1994) Methylation of the oestrogen receptor CpG island links ageing and neoplasia in human colon. Nat Genet 7:536–540
Bennink MR (2001) Dietary soy reduces colon carcinogenesis in human and rats. Soy and colon cancer. Adv Exp Med Biol 492:11–17
Masaoka Y, Watanabe H, Katoh O, Ito A, Dohi K (1998) Effects of miso and NaCl on the development of colonic aberrant crypt foci induced by azoxymethane in F344 rats. Nutr Cancer 32:25–28
Govers MJ, Lapre JA, De Vries HT, Van der Meer R (1993) Dietary soybean protein compared with casein damages colonic epithelium and stimulates colonic epithelial proliferation in rats. J Nutr 123:1709–1713
Hu JF, Zhang SF, Jia EM, Wang QQ, Liu SD, Liu YY, Wu YP, Cheng YT (1988) Diet and cancer of the stomach: a case–control study in China. Int J Cancer 41:331–335
Kono S, Ikeda M, Tokudome S, Kuratsune M (1988) A case–control study of gastric cancer and diet in northern Kyushu, Japan. Jpn J Cancer Res 79:1067–1074
You WC, Blot WJ, Chang YS, Ershow AG, Yang ZT, An Q, Henderson B, Xu GW, Fraumeni JF Jr, Wang TG (1988) Diet and high risk of stomach cancer in Shandong, China. Cancer Res 48:3518–3523
Kato I, Tominaga S, Ito Y, Kobayashi S, Yoshii Y, Matsuura A, Kameya A, Kano T (1990) A comparative case–control analysis of stomach cancer and atrophic gastritis. Cancer Res 50:6559–6564
Hoshiyama Y, Sasaba T (1992) A case–control study of stomach cancer and its relation to diet, cigarettes, and alcohol consumption in Saltama Prefecture, Japan. Cancer Causes Control 3:441–448
Hoshiyama Y, Sekine T, Sasaba T (1993) A case–control study of colorectal cancer and its relation to diet, cigarettes, and alcohol consumption in Saitama Prefecture, Japan. Tohoku J Exp Med 171:153–165
Inoue M, Tajima K, Hirose K, Kuroishi T, Gao CM, Kitoh T (1994) Life-style and subsite of gastric cancer–joint effect of smoking and drinking habits. Int J Cancer 56:494–499
Inoue M, Tajima K, Hirose K, Hamajima N, Takezaki T, Hirai T, Kato T, Ohno Y (1995) Subsite-specific risk factors for colorectal cancer: a hospital-based case–control study in Japan. Cancer Causes Control 6:14–22
Lee JK, Park BJ, Yoo KY, Ahn YO (1995) Dietary factors and stomach cancer: a case–control study in Korea. Int J Epidemiol 24:33–41
Le Marchand L, Hankin JH, Wilkens LR, Kolonel LN, Englyst HN, Lyu LC (1997) Dietary fiber and colorectal cancer risk. Epidemiology 8:658–665
Nishi M, Yoshida K, Hirata K, Miyake H (1997) Eating habits and colorectal cancer. Oncol Rep 4:995–998
Ji BT, Chow WH, Yang G, McLaughlin JK, Zheng W, Shu XO, Jin F, Gao RN, Gao YT, Fraumeni JF Jr (1998) Dietary habits and stomach cancer in Shanghai, China. Int J Cancer 76:659–664
Gao CM, Takezaki T, Ding JH, Li MS, Tajima K (1999) Protective effect of allium vegetables against both esophageal and stomach cancer: a simultaneous case-referent study of a high-epidemic area in Jiangsu Province, China. Jpn J Cancer Res 90:614–621
Huang XE, Hirose K, Wakai K, Matsuo K, Ito H, Xiang J, Takezaki T, Tajima K (2004) Comparison of lifestyle risk factors by family history for gastric, breast, lung and colorectal cancer. APJCP 5:419–427
Ravasco P, Monteiro-Grillo I, Marques Vidal P, Camilo M (2005) Nutritional risks and colorectal cancer in a Portuguese population. Nutr Hosp 20:165–172
Cotterchio M, Boucher BA, Manno M, Gallinger S, Okey A, Harper P (2006) Dietary phytoestrogen intake is associated with reduced colorectal cancer risk. J Nutr 136:3046–3053
Ho SY, Schooling M, Hui LL, McGhee SM, Mak KH, Lam TH (2006) Soy consumption and mortality in Hong Kong: proxy-reported case–control study of all older adult deaths in 1998. Prev Med 43:20–26
Rossi M, Negri E, Talamini R, Bosetti C, Parpinel M, Gnagnarella P, Franceschi S, Dal Maso L, Montella M, Giacosa A, La Vecchia C (2006) Flavonoids and colorectal cancer in Italy. Cancer Epidemiol Biomarkers Prev 15:1555–1558
Williams CD, Satia JA, Adair LS, Stevens J, Galanko J, Keku TO, Sandler RS (2009) Dietary patterns, food groups, and rectal cancer risk in Whites and African–Americans. Cancer Epidemiol Biomarkers Prev 18:1552–1561
Zhang YW, Eom SY, Kim YD, Song YJ, Yun HY, Park JS, Youn SJ, Kim BS, Kim H, Hein DW (2009) Effects of dietary factors and the NAT2 acetylator status on gastric cancer in Koreans. Int J Cancer 125:139–145
Ko KP, Park SK, Park B, Yang JJ, Cho LY, Kang C, Kim CS, Gwack J, Shin A, Kim Y, Kim J, Yang HK, Kang D, Chang SH, Shin HR, Yoo KY (2010) Isoflavones from phytoestrogens and gastric cancer risk: a nested case–control study within the Korean Multicenter Cancer Cohort. Cancer Epidemiol Biomarkers Prev 19:1292–1300
Budhathoki S, Joshi AM, Ohnaka K, Yin G, Toyomura K, Kono S, Mibu R, Tanaka M, Kakeji Y, Maehara Y, Okamura T, Ikejiri K, Futami K, Maekawa T, Yasunami Y, Takenaka K, Ichimiya H, Terasaka R (2011) Soy food and isoflavone intake and colorectal cancer risk: the Fukuoka colorectal cancer study. Scand J Gastroenterol 46:165–172
Nomura A, Grove JS, Stemmermann GN, Severson RK (1990) A prospective study of stomach cancer and its relation to diet, cigarettes, and alcohol consumption. Cancer Res 50:627–631
Kato I, Tominaga S, Matsumoto K (1992) A prospective study of stomach cancer among a rural Japanese population: a 6-year survey. Jpn J Cancer Res 83:568–575
Inoue M, Tajima K, Kobayashi S, Suzuki T, Matsuura A, Nakamura T, Shirai M, Nakamura S, Inuzuka K, Tominaga S (1996) Protective factor against progression from atrophic gastritis to gastric cancer–data from a cohort study in Japan. Int J Cancer 66:309–314
Galanis DJ, Kolonel LN, Lee J, Nomura A (1998) Intakes of selected foods and beverages and the incidence of gastric cancer among the Japanese residents of Hawaii: a prospective study. Int J Epidemiol 27:173–180
Nagata C, Takatsuka N, Kawakami N, Shimizu H (2002) A prospective cohort study of soy product intake and stomach cancer death. Br J Cancer 87:31–36
Ngoan LT, Mizoue T, Fujino Y, Tokui N, Yoshimura T (2002) Dietary factors and stomach cancer mortality. Br J Cancer 87:37–42
Ito LS, Inoue M, Tajima K, Yamamura Y, Kodera Y, Hirose K, Takezaki T, Hamajima N, Kuroishi T, Tominaga S (2003) Dietary factors and the risk of gastric cancer among Japanese women: a comparison between the differentiated and non-differentiated subtypes. Ann Epidemiol 13:24–31
Khan MM, Goto R, Kobayashi K, Suzumura S, Nagata Y, Sonoda T, Sakauchi F, Washio M, Mori M (2004) Dietary habits and cancer mortality among middle aged and older Japanese living in hokkaido, Japan by cancer site and sex. APJCP 5:58–65
Iso H, Kubota Y, Japan Collaborative Cohort Study for Evaluation of C (2007) Nutrition and disease in the Japan collaborative cohort study for evaluation of cancer (JACC). APJCP 8(Suppl):35–80
Sauvaget C, Lagarde F, Nagano J, Soda M, Koyama K, Kodama K (2005) Lifestyle factors, radiation and gastric cancer in atomic-bomb survivors (Japan). Cancer Causes Control 16:773–780
Tokui N, Yoshimura T, Fujino Y, Mizoue T, Hoshiyama Y, Yatsuya H, Sakata K, Kondo T, Kikuchi S, Toyoshima H, Hayakawa N, Kubo T, Tamakoshi A, Group JS (2005) Dietary habits and stomach cancer risk in the JACC Study. J Epidemiol 15(Suppl 2):S98–S108
Oba S, Nagata C, Shimizu N, Shimizu H, Kametani M, Takeyama N, Ohnuma T, Matsushita S (2007) Soy product consumption and the risk of colon cancer: a prospective study in Takayama, Japan. Nutr Cancer 57:151–157
Akhter M, Inoue M, Kurahashi N, Iwasaki M, Sasazuki S, Tsugane S, Japan Public Health Center-Based Prospective Study G (2008) Dietary soy and isoflavone intake and risk of colorectal cancer in the Japan public health center-based prospective study. Cancer Epidemiol Biomarkers Prev 17:2128–2135
Butler LM, Wang R, Koh WP, Yu MC (2008) Prospective study of dietary patterns and colorectal cancer among Singapore Chinese. Br J Cancer 99:1511–1516
Yang G, Shu XO, Li H, Chow WH, Cai H, Zhang X, Gao YT, Zheng W (2009) Prospective cohort study of soy food intake and colorectal cancer risk in women. Am J Clin Nutr 89:577–583
Hara A, Sasazuki S, Inoue M, Iwasaki M, Shimazu T, Sawada N, Yamaji T, Tsugane S, Japan Public Health Center-Based Prospective Study G (2012) Isoflavone intake and risk of gastric cancer: a population-based prospective cohort study in Japan. Am J Clin Nutr 95:147–154
Ko KP, Park SK, Yang JJ, Ma SH, Gwack J, Shin A, Kim Y, Kang D, Chang SH, Shin HR, Yoo KY (2013) Intake of soy products and other foods and gastric cancer risk: a prospective study. J Epidemiol 23:337–343
Kweon SS, Shu XO, Xiang Y, Cai H, Yang G, Ji BT, Li H, Gao YT, Zheng W, Epplein M (2013) Intake of specific nonfermented soy foods may be inversely associated with risk of distal gastric cancer in a Chinese population. J Nutr 143:1736–1742
Willet WC, Howe GR, Kushi LH (1997) Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 65:1220S–1228S
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tse, G., Eslick, G.D. Soy and isoflavone consumption and risk of gastrointestinal cancer: a systematic review and meta-analysis. Eur J Nutr 55, 63–73 (2016). https://doi.org/10.1007/s00394-014-0824-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-014-0824-7