Abstract
Purpose
To investigate the short- and long-term effects of a maternal low-energy diet ad libitum during gestation and/or lactation on mothers and their offspring.
Methods
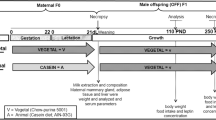
Male Wistar rats were divided into four groups according to their mother’s diet: control [C (19.0 % protein, 63.0 % carbohydrates and 18.0 % lipids, total energetic value (TEV) = 3.5 kcal/g) during gestation and lactation], low-energy diet (18 % protein, 64 % carbohydrates and 18 % lipids, TEV = 2.3 kcal/g) during gestation (LE-G), low-energy diet during lactation (LE-L) and low-energy diet during gestation and lactation (LE-GL). Additional crude fibers (10 % more purified cellulose and soluble fiber) and water (approximately 30 % greater moisture) were added to the LE diet to decrease TEV. Mother’s body weight, food intake and energy intake were recorded daily. Birth weight, growth rate, ontogeny of reflexes, physical features and biochemical parameters at 150 days old were evaluated in male offspring.
Results
Maternal low-energy diet during gestation did not affect maternal body weight and food intake. Physical features did not change but reflex ontogeny was delayed in pups from LE dams. LE-G offspring recovered body size (weight and length) while animals LE-L and LE-GL recovered their body length but remained lighter until adult life even with no change of food intake. LE-G and LE-GL showed lower plasma triglycerides and very-low-density-lipoprotein cholesterol (VLDL). LE-L offspring showed hypertriglyceridemia, high VLDL-c and reduced glycaemia.
Conclusion
Maternal low-energy diet shows discernible short- and long-term effects on offspring, and this is dependent on the time of perinatal period.


Similar content being viewed by others
References
Black RE, Allen LH, Bhutta ZA, Caulfield LE, de Onis M, Ezzati M, Mathers C, Rivera J, Maternal, Child Undernutrition Study G (2008) Maternal and child undernutrition: global and regional exposures and health consequences. Lancet 371:243–260
King JC (2003) The risk of maternal nutritional depletion and poor outcomes increases in early or closely spaced pregnancies. J Nutr 133:1732S–1736S
Senbanjo IO, Olayiwola IO, Afolabi WA, Senbanjo OC (2013) Maternal and child under-nutrition in rural and urban communities of Lagos state, Nigeria: the relationship and risk factors. BMC Res Notes 6:286
Young JB (2006) Developmental origins of obesity: a sympathoadrenal perspective. Int J Obes (Lond) 30(Suppl 4):S41–S49
Barker DJ (1991) The intrauterine environment and adult cardiovascular disease. Ciba Found Symp 156:3–10 (discussion 10-16)
Barker DJ, Eriksson JG, Forsen T, Osmond C (2002) Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol 31:1235–1239
Grijalva-Eternod CS, Wells JC, Cortina-Borja M, Salse-Ubach N, Tondeur MC, Dolan C, Meziani C, Wilkinson C, Spiegel P, Seal AJ (2012) The double burden of obesity and malnutrition in a protracted emergency setting: a cross-sectional study of Western sahara refugees. PLoS Med 9:e1001320
Leandro CG, da Silva Ribeiro W, Dos Santos JA, Bento-Santos A, Lima-Coelho CH, Falcao-Tebas F, Lagranha CJ, Lopes-de-Souza S, Manhaes-de-Castro R, Toscano AE (2012) Moderate physical training attenuates muscle-specific effects on fibre type composition in adult rats submitted to a perinatal maternal low-protein diet. Eur J Nutr 51:807–815
Barros KM, Manhaes-De-Castro R, Lopes-De-Souza S, Matos RJ, Deiro TC, Cabral-Filho JE, Canon F (2006) A regional model (Northeastern Brazil) of induced mal-nutrition delays ontogeny of reflexes and locomotor activity in rats. Nutr Neurosci 9:99–104
Lee S, Lee KA, Choi GY, Desai M, Lee SH, Pang MG, Jo I, Kim YJ (2013) Feed restriction during pregnancy/lactation induces programmed changes in lipid, adiponectin and leptin levels with gender differences in rat offspring. J Matern Fetal Neona Med 26:908–914
West-Eberhard MJ (2005) Phenotypic accommodation: adaptive innovation due to developmental plasticity. J Exp Zool B Mol Dev Evol 304:610–618
Lopes de Souza S, Orozco-Solis R, Grit I, Manhaes de Castro R, Bolanos-Jimenez F (2008) Perinatal protein restriction reduces the inhibitory action of serotonin on food intake. Eur J Neurosci 27:1400–1408
da Silva AA, Borba TK, de Almeida LL, Cavalcante TC, de Freitas MF, Leandro CG, do Nascimento E, de Souza SL (2013) Perinatal undernutrition stimulates seeking food reward. Int J Dev Neurosci 31:334–341
de Brito Alves JL, Nogueira VO, de Oliveira GB, da Silva GS, Wanderley AG, Leandro CG, Costa-Silva JH (2013) Short- and long-term effects of a maternal low-protein diet on ventilation, O2/CO2 chemoreception and arterial blood pressure in male rat offspring. Br J Nutr 111:606–615
de Melo Montenegro IH, Moita L, Dos Reis FK, de Oliveira E, Lisboa PC, de Moura EG, Manhaes-de-Castro R, Leandro CG (2012) Effects of a moderate physical training on the leptin synthesis by adipose tissue of adult rats submitted to a perinatal low-protein diet. Horm Metab Res 44:414–418
Falcao-Tebas F, Bento-Santos A, Fidalgo MA, de Almeida MB, dos Santos JA, Lopes de Souza S, Manhaes-de-Castro R, Leandro CG (2012) Maternal low-protein diet-induced delayed reflex ontogeny is attenuated by moderate physical training during gestation in rats. Br J Nutr 107:372–377
Rehan VK, Sakurai R, Li Y, Karadag A, Corral J, Bellusci S, Xue YY, Belperio J, Torday JS (2012) Effects of maternal food restriction on offspring lung extracellular matrix deposition and long term pulmonary function in an experimental rat model. Pediatr Pulmonol 47:162–171
Sebaai N, Lesage J, Breton C, Vieau D, Deloof S (2004) Perinatal food deprivation induces marked alterations of the hypothalamo-pituitary-adrenal axis in 8-month-old male rats both under basal conditions and after a dehydration period. Neuroendocrinology 79:163–173
Shin BC, Dai Y, Thamotharan M, Gibson LC, Devaskar SU (2012) Pre- and postnatal calorie restriction perturbs early hypothalamic neuropeptide and energy balance. J Neurosci Res 90:1169–1182
Woodall SM, Johnston BM, Breier BH, Gluckman PD (1996) Chronic maternal undernutrition in the rat leads to delayed postnatal growth and elevated blood pressure of offspring. Pediatr Res 40:438–443
Lesage J, Hahn D, Leonhardt M, Blondeau B, Breant B, Dupouy JP (2002) Maternal undernutrition during late gestation-induced intrauterine growth restriction in the rat is associated with impaired placental GLUT3 expression, but does not correlate with endogenous corticosterone levels. J Endocrinol 174:37–43
Vickers MH, Breier BH, Cutfield WS, Hofman PL, Gluckman PD (2000) Fetal origins of hyperphagia, obesity, and hypertension and postnatal amplification by hypercaloric nutrition. Am J Physiol Endocrinol Metab 279:E83–E87
Theys N, Ahn MT, Bouckenooghe T, Reusens B, Remacle C (2011) Maternal malnutrition programs pancreatic islet mitochondrial dysfunction in the adult offspring. J Nutr Biochem 22:985–994
Nascimento E, Muniz GS, de Muniz MGS, Alexandre LS, Rocha LS, Leandro CG, Manhães-de-Castro R, Bolanos-Jimenez F (2013) Unlimited access to low-energy diet causes acute malnutrition in pregnant dams and alters biometric and biochemical parameters in offspring. J Dev Orig Health Dis 5:45–55
Bayne K (1996) Revised guide for the care and use of laboratory animals available. Am Physiol Soc Physiol 39(199):208–211
de Santana Muniz G, da Silva AM, Cavalcante TC, da Silva Franca AK, Ferraz KM, do Nascimento E (2013) Early physical activity minimizes the adverse effects of a low-energy diet on growth and development parameters. Nutr Neurosci 16:113–124
Smart JL, Dobbing J (1971) Vulnerability of developing brain. II. Effects of early nutritional deprivation on reflex ontogeny and development of behaviour in the rat. Brain Res 28:85–95
Novelli EL, Diniz YS, Galhardi CM, Ebaid GM, Rodrigues HG, Mani F, Fernandes AA, Cicogna AC, Novelli Filho JL (2007) Anthropometrical parameters and markers of obesity in rats. Lab Anim 41:111–119
Friedewald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18:499–502
Palou M, Priego T, Sanchez J, Palou A, Pico C (2012) Metabolic programming of sirtuin 1 (SIRT1) expression by moderate energy restriction during gestation in rats may be related to obesity susceptibility in later life. Br J Nutr 109:757–764
Gama EF, Liberti EA, de Souza RR (2007) Effects of pre- and postnatal protein deprivation on atrial natriuretic peptide- (ANP-) granules of the right auricular cardiocytes. An ultrastructural morphometric study. Eur J Nutr 46:245–250
Touyarou P, Sulmont-Rosse C, Issanchou S, Despalins R, Brondel L (2011) Influence of substrate oxidation on the reward system, no role of dietary fibre. Appetite 57:134–141
Ramonet Y, van Milgen J, Dourmad JY, Dubois S, Meunier-Salaun MC, Noblet J (2000) The effect of dietary fibre on energy utilisation and partitioning of heat production over pregnancy in sows. Br J Nutr 84:85–94
Burton-Freeman B (2000) Dietary fiber and energy regulation. J Nutr 130:272S–275S
Bartha L, Nagy GM, Kiem DT, Fekete MI, Makara GB (1991) Inhibition of suckling-induced prolactin release by dexamethasone. Endocrinology 129:635–640
McGowan CA, Walsh JM, Byrne J, Curran S, McAuliffe FM (2013) The influence of a low glycemic index dietary intervention on maternal dietary intake, glycemic index and gestational weight gain during pregnancy: a randomized controlled trial. Nutr J 12:140
Ozanne SE (2001) Metabolic programming in animals. Br Med Bull 60:143–152
Anselmo CW, Silva TL, Holanda TG, Prado LV, Cabral-Filho JE, Catanho MT, Medeiros MC (2008) Influence of a 60 Hz, 3 microT, electromagnetic field on the somatic maturation of wistar rat offspring fed a regional basic diet during pregnancy. Braz J Biol 68:641–648
Lopes AA, Tani G, Katzmarzyk PT, Thomis MA, Maia JA (2014) Association between birth weight and neuromotor performance: a twin study. Scand J Med Sci Sports 24(3):e140–e147
Desai M, Gayle D, Babu J, Ross MG (2007) The timing of nutrient restriction during rat pregnancy/lactation alters metabolic syndrome phenotype. Am J Obstet Gynecol 196(555):e551–e557
Acknowledgments
The authors are indebted for the technical assistance of Lucia Pires and Edeones França. This research was supported by the Foundation for Support of Science and Research in Pernambuco State—Brazil (FACEPE) and the National Council for Research—Brazil (CNPq, 476841/2010-0). The authors’ contributions are as follows: M.C.A.-L., E.N. and C.G.L. performed the study design. L.L.A., N.G.V.-T., E.M.S.-S, T.C.F. and G.S. performed the experiments and data collection. M.C.A.-L. and C.G.L. performed statistical analyses and interpretation of the data. M.C.A.-L. and C.G.L. wrote the manuscript. M.C.A.-L., E.N. and C.G.L. were responsible for critical revisions to the manuscript, and all authors approved the final version. The authors are not aware of any conflicts of interest.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
All authors listed are eligible for authorship.
Rights and permissions
About this article
Cite this article
Alheiros-Lira, M.C., Araújo, L.L., Trindade, N.G.V. et al. Short- and long-term effects of a maternal low-energy diet ad libitum during gestation and/or lactation on physiological parameters of mothers and male offspring. Eur J Nutr 54, 793–802 (2015). https://doi.org/10.1007/s00394-014-0758-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-014-0758-0