Abstract
Purpose
In vitro studies discovered intestinal proton-coupled folate transporter (PCFT) as a vitamin D hormone-responsive gene. In vivo effects of vitamin D on PCFT and folate status are currently not available.
Methods
Three experiments were conducted. At first, vitamin D receptor knockout (VDR−/−) mice and corresponding wild-type (WT) mice were compared for their plasma and hepatic folate concentration and PCFT mRNA expression in intestinal mucosa. In a second experiment with rats, we analyzed the folate status of offspring in response to a maternal vitamin D-adequate (1,000 IU/kg) or vitamin D-deficient (0 IU/kg) diet that was fed for 11 weeks. Finally, the plasma folate concentration of healthy individuals was studied at baseline (in winter) and in response to an oral treatment for 8 weeks with 2,000 IU vitamin D3 per day or a placebo, respectively.
Results
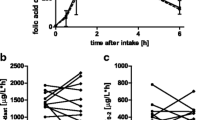
Here, we show that folate status and intestinal PCFT mRNA abundance did not differ between the VDR−/− and the WT mice. No effect of vitamin D on folate status was also found in rat dams and their offspring, and plasma folate levels of individuals did not change in response to vitamin D.
Conclusions
Current data from studies with model animals and humans provide no indication for a vitamin D effect on intestinal uptake and status of folate.
Similar content being viewed by others
References
Holick MF, Chen TC (2008) Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr 87:1080S–1086S
Genuis SJ, Schwalfenberg GK, Hiltz MN, Vaselenak SA (2009) Vitamin D status of clinical practice populations at higher latitudes: analysis and applications. Int J Environ Res Public Health 6:151–173
Holick MF (2006) High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc 81:353–373
Pike JW, Zella LA, Meyer MB, Fretz JA, Kim S (2007) Molecular actions of 1,25-dihydroxyvitamin D3 on genes involved in calcium homeostasis. J Bone Miner Res 22(Suppl 2):V16–V19
Christakos S (2012) Mechanism of action of 1,25-dihydroxyvitamin D3 on intestinal calcium absorption. Rev Endocr Metab Disord 13:39–44
Nagpal S, Na S, Rathnachalam R (2005) Noncalcemic actions of vitamin D receptor ligands. Endocr Rev 26:662–687
Penna G, Roncari A, Amuchastegui S, Daniel KC, Berti E, Colonna M, Adorini L (2005) Expression of the inhibitory receptor ILT3 on dendritic cells is dispensable for induction of CD4 + Foxp3 + regulatory T cells by 1,25-dihydroxyvitamin D3. Blood 106:3490–3497
Wang TT, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J, Tavera-Mendoza L, Lin R, Hanrahan JW, Mader S, White JH (2004) Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol 173:2909–2912
Hansdottir S, Monick MM, Hinde SL, Lovan N, Look DC, Hunninghake GW (2008) Respiratory epithelial cells convert inactive vitamin D to its active form: potential effects on host defense. J Immunol 181:7090–7099
McCarthy TC, Li X, Sinal CJ (2005) Vitamin D receptor-dependent regulation of colon multidrug resistance-associated protein 3 gene expression by bile acids. J Biol Chem 280:23232–23242
Eloranta JJ, Zaïr ZM, Hiller C, Häusler S, Stieger B, Kullak-Ublick GA (2009) Vitamin D3 and its nuclear receptor increase the expression and activity of the human proton-coupled folate transporter. Mol Pharmacol 76:1062–1071
Qiu A, Jansen M, Sakaris A, Min SH, Chattopadhyay S, Tsai E, Sandoval C, Zhao R, Akabas MH, Goldman ID (2006) Identification of an intestinal folate transporter and the molecular basis for hereditary folate malabsorption. Cell 127:917–928
Subramanian VS, Marchant JS, Said HM (2008) Apical membrane targeting and trafficking of the human proton-coupled transporter in polarized epithelia. Am J Physiol Cell Physiol 294:C233–C240
Zhao R, Gao F, Hanscom M, Goldman ID (2004) A prominent low-pH methotrexate transport activity in human solid tumors: contribution to the preservation of methotrexate pharmacologic activity in HeLa cells lacking the reduced folate carrier. Clin Cancer Res 10:718–727
Wang Y, Rajgopal A, Goldman ID, Zhao R (2005) Preservation of folate transport activity with a low-pH optimum in rat IEC-6 intestinal epithelial cell lines that lack reduced folate carrier function. Am J Physiol Cell Physiol 288:C65–C71
Max D, Brandsch C, Schumann S, Kühne H, Frommhagen F, Schutkowski A, Hirche F, Staege MS, Stangl GI (2013) Maternal vitamin D deficiency causes smaller muscle fibers and altered transcript levels of genes involved in protein degradation, myogenesis, and cytoskeleton organization in the newborn rat. Mol Nutr Food Res. doi:10.1002/mnfr.201300360
Lehmann U, Hirche F, Stangl GI, Hinz K, Westphal S, Dierkes J (2013) Bioavailability of vitamin D2 and D3 in healthy volunteers, a randomised placebo-controlled trial. J Clin Endocrinol Metab. doi:10.1210/jc.2012-4287
National Research Council (NRC), Committee on Animal Nutrition (1995) Nutrient Requirements of Laboratory Animals. National Academy Press, Washington, DC
Reeves PG, Nielsen FH, Fahey GC Jr (1993) AIN-93 purified diets for laboratory rodents: final report of the American institute of nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 123:1939–1951
Schmidt N, Brandsch C, Kühne H, Thiele A, Hirche F, Stangl GI (2012) Vitamin D receptor deficiency and low vitamin D diet stimulate aortic calcification and osteogenic key factor expression in mice. PLoS ONE 7:e35316
Untersuchung von Lebensmitteln: Bestimmung von Vitamin D (Cholecalciferol (D3) und Ergocalciferol (D2)) in Lebensmitteln mittels HPLC. L 00.00-61, Juli 2001, in: Amtliche Sammlung von Untersuchungsverfahren nach §35 LMBG, Bundesgesundheitsamt (ed), Beuth, Berlin, Köln, (according to DIN EN12821)
Mattila PH, Piironen VI, Uusi-Rauva EJ, Koivistoinen PE (1995) Contents of cholecalciferol, ergocalciferol, and their 25-hydroxylated metabolites in milk products and raw meat and liver as determined by HPLC. J Agric Food Chem 43:2394–2399
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45
Kondo A, Kamihira O, Ozawa H (2009) Neural tube defects: prevalence, etiology and prevention. Int J Urol 16:49–57
Flood VM, Smith WT, Webb KL, Rochtchina E, Anderson VE, Mitchell P (2006) Prevalence of low serum folate and vitamin B12 in an older Australian population. Aust NZ J Public Health 30:38–41
Qiu A, Min SH, Jansen M, Malhotra U, Tsai E, Cabelof DC, Matherly LH, Zhao R, Akabas MH, Goldman ID (2007) Rodent intestinal folate transporters (SLC46A1): secondary structure, functional properties, and response to dietary folate restriction. Am J Physiol Cell Physiol 293:C1669–C1678
Zhao R, Qiu A, Tsai E, Jansen M, Akabas MH, Goldman ID (2008) The proton-coupled folate transporter: impact on pemetrexed transport and on antifolates activities compared with the reduced folate carrier. Mol Pharmacol 74:854–862
Lips P (2007) Relative value of 25(OH)D and 1,25(OH)2D measurements. J Bone Miner Res 22:1668–1671
Gonen N, Assaraf YG (2010) The obligatory intestinal folate transporter PCFT (SLC46A1) is regulated by nuclear respiratory factor 1. J Biol Chem 285:33602–33613
Gonen N, Bram EE, Assaraf YG (2008) PCFT/SLC46A1 promoter methylation and restoration of gene expression in human leukemia cells. Biochem Biophys Res Commun 376:787–792
Wasson GR, McGlynn AP, McNulty H, O’Reilly SL, McKelvey-Martin VJ, McKerr G, Strain JJ, Scott J, Downes CS (2006) Global DNA and p53 region-specific hypomethylation in human colonic cells is induced by folate depletion and reversed by folate supplementation. J Nutr 136:2748–2753
Zhao R, Diop-Bove N, Visentin M, Goldman ID (2011) Mechanisms of membrane transport of folates into cells and across epithelia. Annu Rev Nutr 31:177–201
Borradale DC, Kimlin MG (2012) Folate degradation due to ultraviolet radiation: possible implications for human health and nutrition. Nutr Rev 70:414–422
Cicarma E, Mørk C, Porojnicu AC, Juzeniene A, Tam TT, Dahlback A, Moan J (2010) Influence of narrowband UVB phototherapy on vitamin D and folate status. Exp Dermatol 19:e67–e72
Juzeniene A, Stokke KT, Thune P, Moan J (2010) Pilot study of folate status in healthy volunteers and in patients with psoriasis before and after UV exposure. J Photochem Photobiol B 101:111–116
Acknowledgments
Parts of this work were supported by Grant 0315668A from the Federal Ministry of Education and Research of Germany.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brandsch, C., Zibolka, J., Frommhagen, M. et al. Vitamin D is not linked to folate status and mRNA expression of intestinal proton-coupled folate transporter. Eur J Nutr 53, 1115–1122 (2014). https://doi.org/10.1007/s00394-013-0614-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-013-0614-7