Abstract
Background
Heart produces ATP through long-chain fatty acids beta oxidation.
Purpose
To analyze whether in ventricular myocardium, high-fat diet may modify the expression of proteins associated with energy metabolism before myocardial function was affected.
Methods
Wistar Kyoto rats were divided into two groups: (a) rats fed standard diet (control; n = 6) and (b) rats fed high-fat diet (HFD; n = 6). Proteins from left ventricles were analyzed by two-dimensional electrophoresis, mass spectrometry and Western blotting.
Results
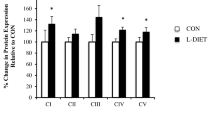
Rats fed with HFD showed higher body weight, insulin, glucose, leptin and total cholesterol plasma levels as compared with those fed with standard diet. However, myocardial functional parameters were not different between them. The protein expression of 3-ketoacyl-CoA thiolase, acyl-CoA hydrolase mitochondrial precursor and enoyl-CoA hydratase, three long-chain fatty acid β-oxidation-related enzymes, and carnitine-O-palmitoyltransferase I was significantly higher in left ventricles from HFD rats. Protein expression of triosephosphate isomerase was higher in left ventricles from HFD rats than in those from control. Two α/β-enolase isotypes and glyceraldehyde-3-phosphate isomerase were significantly increased in HFD rats as compared with control. Pyruvate and lactate contents were similar in HFD and control groups. Expression of proteins associated with Krebs cycle and mitochondrial oxidative phosphorylation was higher in HFD rats.
Conclusions
Expression of proteins involved in left ventricle metabolic energy was enhanced before myocardial functionality was affected in rats fed with HFD. These findings may probably indicate higher cardiac energy requirement due to weight increase by HFD.


Similar content being viewed by others
References
Taegtmeyer H (1994) Energy metabolism of the heart: from basic concepts to clinical applications. Curr Probl Cardiol 19:59–113
Krauss RM, Eckel RH, Howard B, Appel LJ, Daniels SR, Deckelbaum RJ, Erdman JW Jr, Kris-Etherton P, Goldberg IJ, Kotchen TA (2000) AHA dietary guidelines. A statement for healthcare professionals from the nutrition committee of the American Heart Association. Circulation 102:2284–2299. doi:10.1161/01.CIR.102.18.2284
Shigematsu Y, Hara Y, Ohtsuka T, Ohgimoto A, Inoue K, Higaki J (2005) Relation of genetic predisposition and insulin resistance to left ventricular hypertrophy in hypertension. Am J Hypertens 18:457–463. doi:10.1016/j.amjhyper.2004.10.027
Panchal SK, Poudyal H, Iver A, Nazer R, Alam A, Diwan V, Kauter K, Sernia C, Campbell F, Ward L (2011) High-carbohydrate high-fat diet-induced metabolic syndrome and cardiovascular remodelling in rats. J Cardiovasc Pharmacol 57:51–64. doi:10.1097/FJC.0b013e3181feb90a
Zamorano-León JJ, Modrego J, Mateos-Cáceres PJ, Macaya C, Martín-Fernández B, Miana M, de las Heras N, Cachofeiro V, Lahera V, López-Farré AJ (2010) A proteomic approach to determine changes in proteins involved in the myocardial metabolism in left ventricles of spontaneously hypertensive rats. Cell Physiol Biochem 25:347–358
McGill JB, Peterson LR, Herrero P, Saeed IM, Recklein C, Coggan AR, Demoss AJ, Schechtman KB, Dence CS, Gropler RJ (2011) Potentiation of abnormalities in myocardial metabolism with the development of diabetes in women with obesity and insulin resistance. J Nucl Cardiol 18(3):421–429. doi:10.1007/s12350-011-9362-3
Chiu HC, Kovacs A, Ford DA, Hsu FF, García R, Herrero P, Saffitz JE, Schaffer JE (2001) A novel mouse model of lipotoxic cardiomyopathy. J Clin Invest 107:813–822. doi:10.1172/JCI10947
Wang P, Lloyd SG, Zeng H, Bonen A, Chatham JC (2005) Impact of altered substrate utilization on cardiac function in isolated hearts from Zucker diabetic fatty rats. Am J Physiol Heart Circ Physiol 288:H2102–H2110. doi:10.1152/ajpheart.00935.2004
Peterson LR, Herrero P, Schechtman KB, Racette SB, Waggoner AD, Kisrieva-Ware Z, Dence C, Klein S, Marsala J, Meyer T (2004) Effect of obesity and insulin resistance on myocardial substrate metabolism and efficiency in young women. Circulation 109:2191–2196. doi:10.1161/01.CIR.0000127959.28627.F8
Okere IC, Chandler MP, McElfresh TA, Rennison JH, Sharov V, Sabbah HN, Tserng KY, Hoit BD, Ernsberger P, Young ME (2006) Differential effects of saturated and unsaturated fatty acid diets on cardiomyocyte apoptosis, adipose distribution, and serum leptin. Am J Physiol Heart Circ Physiol 291:H38–H44. doi:10.1152/ajpheart.01295.2005
Zhao X, Wu N, Deng M, Yin Y, Zhou J, Fang Y, Huang L (2006) An improved method of left ventricular catheterization in rats. Physiol Meas 27(6):N27–N33. doi:10.1088/0967-3334/27/6/N01
de las Heras N, Martín-Fernández B, Miana M, Ballesteros S, Oubiña MP, López-Farré AJ, Cachofeiro V, Lahera V (2009) The protective effect of irbesartan in rats fed a high fat diet is associated with modification of leptin–adiponectin imbalance. J Hypertens Suppl 27:S37–S41
Mateos-Cáceres PJ, García-Méndez A, López-Farré AJ, Macaya C, Núñez A, Gómez J, Alonso-Orgaz S, Carrasco C, Burgos ME, de Andrés R (2004) Proteomic analysis of plasma from patients during an acute coronary syndrome. J Am Coll Cardiol 44:1578–1583. doi:10.1016/j.jacc.2004.06.073
Molero L, García-Méndez A, Alonso-Orgaz S, Carrasco C, Macaya C, López-Farré AJ (2005) Proteomic approach to identify changes in protein expression modified by 17beta-oestradiol in bovine vascular smooth muscle cells. Clin Sci (Lond) 109:457–463. doi:10.1042/CS20050082
McGarry JD, Foster DW (1980) Regulation of hepatic fatty acid oxidation and ketone body production. Annu Rev Biochem 49:395–420. doi:10.1146/annurev.bi.49.070180.002143
McGarry JD, Woeltje KF, Kuwajima M, Foster DW (1989) Regulation of ketogenesis and the renaissance of carnitine palmitoyltransferase. Diabetes Metab Rev 5:271–284
How O-J, Aasum E, Kunnathu S, Severson DL, Myhre ES, Larsen TS (2005) Influence of substrate supply on cardiac efficiency, as measured by pressure-volume analysis in ex vivo mouse hearts. Am J Physiol Heart Circ physiol 288:H2979–H2985. doi:10.1152/ajpheart.00084.2005
Aasum E, Hafstad AD, Severson DL, Larsen TS (2003) Age-dependent changes in metabolism, contractile function, and ischemic sensitivity in hearts from db/db mice. Diabetes 52:434–441. doi:10.2337/diabetes.52.2.434
Mazumder PK, O’Neill BT, Roberts MW, Buchanan J, Yun UJ, Cooksey RC (2004) Impaired cardiac efficiency and increased fatty acid oxidation in insulin-resistant ob/ob mouse hearts. Diabetes 53:2366–2374. doi:10.2337/diabetes.53.9.2366
Yan J, Young ME, Cui L, Lopaschuk GD, Liao R, Tian R (2009) Increased glucose uptake and oxidation in mouse hearts prevent high fatty acid oxidation but cause cardiac dysfunction in diet-induced obesity. Circulation 119:2818–2828. doi:10.1161/CIRCULATIONAHA.108.832915
Boudina S, Sena S, O’Neill BT, Tathireddy P, Young ME, Abel Dale E (2005) Reduced mitochondrial oxidative capacity and increased mitochondrial uncoupling impair myocardial energetics in obesity. Circulation 112:2686–2695. doi:10.1161/CIRCULATIONAHA.105.554360
Mollica MP, Iossa S, Liverini G, Soboll S (1998) Steady state changes in mitochondrial electrical potential and proton gradient in perfused liver from rats fed a high fat diet. Mol Cell Biochem 178:213–217. doi:10.1023/A:1006899632413
Haap M, Houdali B, Maerker E, Renn W, Machicao F, Hoffmeister HM (2003) Insulin-like effect of low-dose leptin on glucose transport in Langendorff rat heats. Exp Clin Endocrinol Diabetes 111:139–145. doi:10.1055/s-2003-39786
Atkinson LL, Fischer MA, Lopaschuk GD (2002) Leptin activates fatty acid oxidation independent of changes in the AMP-activated protein kinase-acetyl-CoA carboxylase-malonyl-CoA axis. J Biol Chem 277:29424–29430. doi:10.1074/jbc.M203813200
Buchanan J, Mazumder PK, Hu P, Chakrabarti G, Roberts MW, Yun UJ (2005) Reduced cardiac efficiency and altered substrate metabolism precedes the onset of hyperglycemia and contractile dysfunction in two mouse models of insulin resistance and obesity. Endocrinology 146:5341–5349. doi:10.1210/en.2005-0938
Rahmouni K, Correia MLG, Haynes WG, Mark AL (2005) Obesity-associated hypertension: new insights into mechanisms. Hypertension 45:9–14. doi:10.1161/01.HYP.0000151325.83008.b4
Francischetti EA, Genelhu VA (2007) Obesity-hypertension: an ongoing pandemic. Int J Clin Pract 61:269–280. doi:10.1111/j.1742-1241.2006.01262.x
Liu J, Wang P, Luo J, Huang Y, He L, Yang H, Li Q, Wu S, Zhelyabovska O, Yang Q (2011) Peroxisome proliferator-activated receptor β/δ activation in adult hearts facilitates mitochondrial function and cardiac performance under pressure-overload condition. Hypertension 57:223–230. doi:10.1161/HYPERTENSIONAHA.110.164590
Hafstad AD, Khalid AM, Hagve M, Lund T, Larsen TS, Severson DL, Clarke K, Berge RK, Aasum E (2009) Cardiac peroxisome proliferator-activated receptor-alpha activation causes increased fatty acid oxidation, reducing efficiency and post-ischaemic functional loss. Cardiovasc Res 83:519–526. doi:10.1093/cvr/cvp132
Cheng L, Ding G, Qin Q, Huang Y, Lewis W, He N, Evans RM, Schneider MD, Brako FA, Xiao Y, Chen YE, Yang Q (2004) Cardiomyocyte-restricted peroxisome proliferator-activated receptor-delta deletion perturbs myocardial fatty acid oxidation and leads to cardiomyopathy. Nat Med 10:1245–1250. doi:10.1038/nm1116
Brandt JM, Djouadi F, Kelly DP (1998) Fatty acids activate transcription of the muscle carnitine palmitoyltransferase I gene in cardiac myocytes via the peroxisome proliferator-activated receptor alpha. J Biol Chem 273:23786–23792. doi:10.1074/jbc.273.37.23786
Acknowledgments
Javier Modrego and José J. Zamorano-León are staff of Red Heracles. Petra J. Mateos-Cáceres is staff member of Fundación para la Investigación Biomédica, Hospital Clínico San Carlos. The authors thank Begoña Larrea and Miguel Dantart for their secretarial assistance. We also thank Sandra Ballesteros for her technical assistance. This work has been supported by Fondo de Investigaciones de la Seguridad Social (Redes Temáticas de Investigación Cooperativa, Red Heracles RD06/0009/0010) and Fundación Mutua Madrileña.
Conflict of interest
Authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Modrego, J., de las Heras, N., Zamorano-León, J.J. et al. Changes in cardiac energy metabolic pathways in overweighed rats fed a high-fat diet. Eur J Nutr 52, 847–856 (2013). https://doi.org/10.1007/s00394-012-0392-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-012-0392-7