Abstract
Purpose
In addition to genetic risk, environmental factors might influence coeliac disease (CD) development. We sought to assess the effect of the interaction between milk-feeding practices and the HLA-DQ genotype on peripheral lymphocyte subsets and their activation markers in infants at familial risk for CD.
Methods
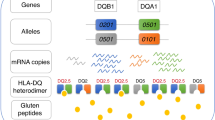
170 newborns were classified in 3 different genetic risk groups (high risk, HR; intermediate risk, IR; and low risk, LR) after DQB1 and DQA1 typing. Lymphocyte subsets were studied at the age of 4 months by flow cytometry analysis.
Results
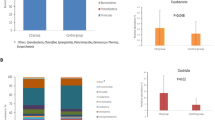
79 infants were receiving exclusive breastfeeding (BF) and 91 partial breastfeeding or formula feeding (FF). Regarding genetic risk, 40 infants were classified in HR group, 75 in IR group and 55 in LR group. Two-way ANOVA did not show significant interactions between the type of milk feeding and genetic risk group on the lymphocyte subsets analysed. One-way ANOVA for milk-feeding practice alone showed that the percentage of CD4 + CD25+ cells was significantly higher in BF group than in FF group (BF, 10.92 ± 2.71; FF, 9.94 ± 2.96; p = 0.026), and absolute counts of CD4 + CD38+ cells were significantly higher in FF group than in BF group (FF, 2,881.23 ± 973.48; BF, 2,557.95 ± 977.06; p = 0.038). One-way ANOVA for genetic risk alone showed that absolute counts of NK cells were significantly higher in IR group than HR and LR groups (IR, 539.24 ± 340.63; HR, 405.01 ± 239.53; LR, 419.86 ± 262.85; p = 0.028).
Conclusion
Lymphocyte subset profiles in the early stages of life could be modulated by milk-feeding practices and genetic risk separately. Breastfeeding might have a positive immunomodulatory effect on lymphocyte subsets in infants at risk of CD.

Similar content being viewed by others
Abbreviations
- CD:
-
Coeliac disease
- PCR-SPP:
-
Polymerase chain reaction-sequence-specific primers
- HLA:
-
Human leucocyte antigen
- Treg:
-
Regulatory T cells
- NK:
-
Natural killer
- LR:
-
Low genetic risk
- IR:
-
Intermediate genetic risk
- HR:
-
High genetic risk
- BF:
-
Breastfeeding
- FF:
-
Formula/mixture feeding
References
Gianfrani C, Auricchio S, Troncone R (2005) Adaptive and innate immune responses in celiac disease. Immunol Lett 99(2):141–145. doi:10.1016/j.imlet.2005.02.017
Dubois PC, van Heel DA (2008) Translational mini-review series on the immunogenetics of gut disease: immunogenetics of coeliac disease. Clin Exp Immunol 153(2):162–173. doi:10.1111/j.1365-2249.2008.03704.x
Kagnoff MF (2007) Celiac disease: pathogenesis of a model immunogenetic disease. J Clin Invest 117(1):41–49. doi:10.1172/JCI30253
Bourgey M, Calcagno G, Tinto N, Gennarelli D, Margaritte-Jeannin P, Greco L, Limongelli MG, Esposito O, Marano C, Troncone R, Spampanato A, Clerget-Darpoux F, Sacchetti L (2007) HLA related genetic risk for coeliac disease. Gut 56(8):1054–1059. doi:10.1136/gut.2006.108530
Karell K, Louka AS, Moodie SJ, Ascher H, Clot F, Greco L, Ciclitira PJ, Sollid LM, Partanen J (2003) HLA types in celiac disease patients not carrying the DQA1*05-DQB1*02 (DQ2) heterodimer: results from the European genetics cluster on celiac disease. Hum Immunol 64(4):469–477
Nova E, Pozo T, Sanz Y, Marcos A (2010) Dietary strategies of immunomodulation in infants at risk for celiac disease. Proc Nutr Soc 69(3):347–353. doi:10.1017/S0029665110001825
Westerbeek EA, van den Berg A, Lafeber HN, Knol J, Fetter WP, van Elburg RM (2006) The intestinal bacterial colonisation in preterm infants: a review of the literature. Clin Nutr 25(3):361–368. doi:10.1016/j.clnu.2006.03.002
Williams AM, Bland PW, Phillips AC, Turner S, Brooklyn T, Shaya G, Spicer RD, Probert CS (2004) Intestinal alpha beta T cells differentiate and rearrange antigen receptor genes in situ in the human infant. J Immunol 173(12):7190–7199
Niers L, Stasse-Wolthuis M, Rombouts FM, Rijkers GT (2007) Nutritional support for the infant’s immune system. Nutr Rev 65(8 Pt 1):347–360
Martin R, Langa S, Reviriego C, Jiminez E, Marin ML, Xaus J, Fernandez L, Rodriguez JM (2003) Human milk is a source of lactic acid bacteria for the infant gut. J Pediatr 143(6):754–758. doi:10.1016/j.jpeds.2003.09.028
Schack-Nielsen L, Michaelsen KF (2007) Advances in our understanding of the biology of human milk and its effects on the offspring. J Nutr 137(2):503S–510S
de Vries E, de Bruin-Versteeg S, Comans-Bitter WM, de Groot R, Hop WC, Boerma GJ, Lotgering FK, van Dongen JJ (2000) Longitudinal survey of lymphocyte subpopulations in the first year of life. Pediatr Res 47(4 Pt 1):528–537
Comans-Bitter WM, de Groot R, van den Beemd R, Neijens HJ, Hop WC, Groeneveld K, Hooijkaas H, van Dongen JJ (1997) Immunophenotyping of blood lymphocytes in childhood. Reference values for lymphocyte subpopulations. J Pediatr 130(3):388–393
Panaro A, Amati A, di Loreto M, Felle R, Ferrante M, Papadia AM, Porfido N, Gambatesa V, Dell’Osso A, Lucivero G (1991) Lymphocyte subpopulations in pediatric age. Definition of reference values by flow cytometry. Allergol Immunopathol Madr 19(3):109–112
Shearer WT, Rosenblatt HM, Gelman RS, Oyomopito R, Plaeger S, Stiehm ER, Wara DW, Douglas SD, Luzuriaga K, McFarland EJ, Yogev R, Rathore MH, Levy W, Graham BL, Spector SA (2003) Lymphocyte subsets in healthy children from birth through 18 years of age: the Pediatric AIDS Clinical Trials Group P1009 study. J Allergy Clin Immunol 112(5):973–980. doi:10.1016/j.jaci.2003.07.003
Jackson DG, Bell JI (1990) Isolation of a cDNA encoding the human CD38 (T10) molecule, a cell surface glycoprotein with an unusual discontinuous pattern of expression during lymphocyte differentiation. J Immunol 144(7):2811–2815
Carver JD, Pimentel B, Wiener DA, Lowell NE, Barness LA (1991) Infant feeding effects on flow cytometric analysis of blood. J Clin Lab Anal 5(1):54–56
Cummins AG, Eglinton BA, Gonzalez A, Roberton DM (1994) Immune activation during infancy in healthy humans. J Clin Immunol 14(2):107–115
Hawkes JS, Neumann MA, Gibson RA (1999) The effect of breast feeding on lymphocyte subpopulations in healthy term infants at 6 months of age. Pediatr Res 45(5 Pt 1):648–651
Jeppesen DL, Hasselbalch H, Lisse IM, Ersboll AK, Engelmann MD (2004) T-lymphocyte subsets, thymic size and breastfeeding in infancy. Pediatr Allergy Immunol 15(2):127–132. doi:10.1111/j.1399-3038.2004.00032.xPAI032
Donat E, Planelles D, Capilla-Villanueva A, Montoro JA, Palau F, Ribes-Koninckx C (2009) Allelic distribution and the effect of haplotype combination for HLA type II loci in the celiac disease population of the Valencian community (Spain). Tissue Antigens 73(3):255–261. doi:10.1111/j.1399-0039.2008.01191.x
Berrington JE, Barge D, Fenton AC, Cant AJ, Spickett GP (2005) Lymphocyte subsets in term and significantly preterm UK infants in the first year of life analysed by single platform flow cytometry. Clin Exp Immunol 140(2):289–292. doi:10.1111/j.1365-2249.2005.02767.x
Peters U, Schneeweiss S, Trautwein EA, Erbersdobler HF (2001) A case-control study of the effect of infant feeding on celiac disease. Ann Nutr Metab 45(4):135–142
Ivarsson A, Hernell O, Stenlund H, Persson LA (2002) Breast-feeding protects against celiac disease. Am J Clin Nutr 75(5):914–921
Branski D, Fasano A, Troncone R (2006) Latest developments in the pathogenesis and treatment of celiac disease. J Pediatr 149(3):295–300. doi:10.1016/j.jpeds.2006.06.003
Kimpimaki T, Erkkola M, Korhonen S, Kupila A, Virtanen SM, Ilonen J, Simell O, Knip M (2001) Short-term exclusive breastfeeding predisposes young children with increased genetic risk of Type I diabetes to progressive beta-cell autoimmunity. Diabetologia 44(1):63–69
McLoughlin RM, Calatroni A, Visness CM, Wallace PK, Cruikshank WW, Tuzova M, Ly NP, Ruiz-Perez B, Kattan M, Bloomberg GR, Lederman H, Gern JE, Gold DR (2011) Longitudinal relationship of early life immunomodulatory T cell phenotype and function to development of allergic sensitization in an urban cohort. Clin Exp Allergy J Br Soc Allergy Clin Immunol. doi:10.1111/j.1365-2222.2011.03882.x
Brugman S, Visser JT, Hillebrands JL, Bos NA, Rozing J (2009) Prolonged exclusive breastfeeding reduces autoimmune diabetes incidence and increases regulatory T-cell frequency in bio-breeding diabetes-prone rats. Diabetes Metab Res Rev 25(4):380–387. doi:10.1002/dmrr.953
Mosconi E, Rekima A, Seitz-Polski B, Kanda A, Fleury S, Tissandie E, Monteiro R, Dombrowicz DD, Julia V, Glaichenhaus N, Verhasselt V (2010) Breast milk immune complexes are potent inducers of oral tolerance in neonates and prevent asthma development. Mucosal Immunol 3(5):461–474. doi:10.1038/mi.2010.23
Lan RY, Ansari AA, Lian ZX, Gershwin ME (2005) Regulatory T cells: development, function and role in autoimmunity. Autoimmun Rev 4(6):351–363. doi:10.1016/j.autrev.2005.01.007
Admyre C, Johansson SM, Qazi KR, Filen JJ, Lahesmaa R, Norman M, Neve EP, Scheynius A, Gabrielsson S (2007) Exosomes with immune modulatory features are present in human breast milk. J Immunol 179(3):1969–1978
Perez-Cano FJ, Dong H, Yaqoob P (2010) In vitro immunomodulatory activity of Lactobacillus fermentum CECT5716 and Lactobacillus salivarius CECT5713: two probiotic strains isolated from human breast milk. Immunobiology 215(12):996–1004. doi:10.1016/j.imbio.2010.01.004
Zhou YJ, Gao J, Yang HM, Yuan XL, Chen TX, He ZJ (2010) The role of the lactadherin in promoting intestinal DCs development in vivo and vitro. Clin Dev Immunol 2010:357541. doi:10.1155/2010/357541
Newburg DS, Peterson JA, Ruiz-Palacios GM, Matson DO, Morrow AL, Shults J, Guerrero ML, Chaturvedi P, Newburg SO, Scallan CD, Taylor MR, Ceriani RL, Pickering LK (1998) Role of human-milk lactadherin in protection against symptomatic rotavirus infection. Lancet 351(9110):1160–1164
Newburg DS (1999) Human milk glycoconjugates that inhibit pathogens. Curr Med Chem 6(2):117–127
Peterson JA, Scallan CD, Ceriani RL, Hamosh M (2001) Structural and functional aspects of three major glycoproteins of the human milk fat globule membrane. Adv Exp Med Biol 501:179–187
Kvistgaard AS, Pallesen LT, Arias CF, Lopez S, Petersen TE, Heegaard CW, Rasmussen JT (2004) Inhibitory effects of human and bovine milk constituents on rotavirus infections. J Dairy Sci 87(12):4088–4096. doi:10.3168/jds.S0022-0302(04)73551-1
Stene LC, Honeyman MC, Hoffenberg EJ, Haas JE, Sokol RJ, Emery L, Taki I, Norris JM, Erlich HA, Eisenbarth GS, Rewers M (2006) Rotavirus infection frequency and risk of celiac disease autoimmunity in early childhood: a longitudinal study. Am J Gastroenterol 101(10):2333–2340. doi:10.1111/j.1572-0241.2006.00741.x
Guandalini S (2007) The influence of gluten: weaning recommendations for healthy children and children at risk for celiac disease. Nestle Nutr Workshop Ser Pediatr Program 60:139–151. doi:10.1159/0000106366 (Discussion 151–135)
Chirdo FG, Rumbo M, Anon MC, Fossati CA (1998) Presence of high levels of non-degraded gliadin in breast milk from healthy mothers. Scandinavian J Gastroenterol 33(11):1186–1192
Rumbo M, Chirdo FG, Anon MC, Fossati CA (1998) Detection and characterization of antibodies specific to food antigens (gliadin, ovalbumin and beta-lactoglobulin) in human serum, saliva, colostrum and milk. Clin Exp Immunol 112(3):453–458
Ozkan T, Ozeke T, Meral A (2000) Gliadin-specific IgA antibodies in breast milk. J Int Med Res 28(5):234–240
Hall MA, Norman PJ, Thiel B, Tiwari H, Peiffer A, Vaughan RW, Prescott S, Leppert M, Schork NJ, Lanchbury JS (2002) Quantitative trait loci on chromosomes 1, 2, 3, 4, 8, 9, 11, 12, and 18 control variation in levels of T and B lymphocyte subpopulations. Am J Hum Genet 70(5):1172–1182. doi:10.1086/340090
Amadori A, Zamarchi R, De Silvestro G, Forza G, Cavatton G, Danieli GA, Clementi M, Chieco-Bianchi L (1995) Genetic control of the CD4/CD8 T-cell ratio in humans. Nat Med 1(12):1279–1283
Clementi M, Forabosco P, Amadori A, Zamarchi R, De Silvestro G, Di Gianantonio E, Chieco-Bianchi L, Tenconi R (1999) CD4 and CD8 T lymphocyte inheritance. Evidence for major autosomal recessive genes. Hum Genet 105(4):337–342
Puglisi F, Capuano P, Simone M, Verzillo F, Laurentaci C (1999) T-cell activity in HLA-associated autoimmune diseases. Panminerva Med 41(4):315–317
Acknowledgments
We gratefully acknowledge the assistance of the statistician Dr. Laura Barrios in the statistical analysis. Supported by grants AGL2007-66126-C03-01/ALI, AGL2007-66126-C03-02/ALI and AGL2007-66126-C03-03/ALI, from the Spanish Ministry of Science and Innovation and grants 200570F0091 and 200570F0093 from CSIC. T. Pozo and G. de Palma were recipients of a personal grant from the JAE/I3P Program of CSIC (Spain).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pozo-Rubio, T., Capilla, A., Mujico, J.R. et al. Influence of breastfeeding versus formula feeding on lymphocyte subsets in infants at risk of coeliac disease: the PROFICEL study. Eur J Nutr 52, 637–646 (2013). https://doi.org/10.1007/s00394-012-0367-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-012-0367-8