Abstract
Purpose
Folate-related nutrient–nutrient and nutrient–gene interactions modify disease risk; we therefore examined synergistic relationships between dietary folic acid, vitamin C and variant folate genes with respect to red cell folate status.
Methods
Two hundred and twelve subjects were examined using chemiluminescent immunoassay, PCR and food frequency questionnaire to determine red cell and serum folate, 14 folate gene polymorphisms, dietary folate (natural and synthetic) and vitamin C.
Results
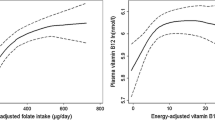
When examined independently, synthetic PteGlu correlates best with red cell folate at higher levels of intake (p = 0.0102), while natural 5CH3-H4-PteGlun correlates best with red cell folate at lower levels of intake (p = 0.0035). However, dietary vitamin C and 5CH3-H4-PteGlun interact synergistically to correlate with red cell folate at higher levels of intake (p = 0.0005). No interaction between dietary vitamin C and PteGlu was observed. This ‘natural’ nutrient–nutrient interaction may provide an alternative to synthetic PteGlu supplementation that is now linked to adverse phenomena/health outcomes. On its own, vitamin C also correlates with red cell folate (p = 0.0150) and is strongly influenced by genetic variation in TS, MTHFR and MSR, genes critical for DNA and methionine biosynthesis that underpin erythropoiesis. Similarly, dietary vitamin C and 5CH3-H4-PteGlun act synergistically to modify red cell folate status according to variation in folate genes: of note, heterozygosity for 2R3R-TS (p = 0.0181), SHMT (p = 0.0046) and all three MTHFR SNPs (p = 0.0023, 0.0015 and 0.0239 for G1793A, C677T and A1298C variants, respectively) promote a significant association with red cell folate. Again, all these genes are critical for nucleic acid biosynthesis. Folate variants with the strongest independent effect on folate status were C677T-MTHFR (p = 0.0004) and G1793A-MTHFR (p = 0.0173).
Conclusions
5CH3-H4-PteGlun assimilation and variant folate gene expression products may be critically dependent on dietary vitamin C.

Similar content being viewed by others
References
Gregory JF III (2001) Case study: folate bioavailability. J Nutr 131:1376–1382
Lucock M (2004) Clinical review science, medicine, and the future: is folic acid the ultimate functional food component for disease prevention? Br Med J 328:211–214
Gregory JF III (1997) Bioavailability of folate. Eur J Clin Nutr 51:54–59
Sauberlich HE, Kretsch MJ, Skalah JH, Johnson HL, Talyor PC (1987) Folate requirement and metabolism in nonpregnant women. Am J Clin Nutr 46:1016–1028
Northwestern University (2004) http://www.feinberg.northwestern.edu/nutrition/factsheets/folate.pdf
Keagy PM (1985) Folacin: microbiological and animal assays. In: Methods of vitamin assay, 4th edn. Wiley, New York, pp 445–471
Blakeley RL (1969) The chemistry of folic acid and related pteridines. Front Biol 13:58–105
Murphy M, Keating M, Boyle P, Weir DG, Scott JM (1976) The elucidation of the mechanism of folate catabolism in the rat. Biochem Biophys Res Commun 71:1017–1024
Murphy M, Boyle PHM, Weir DG, Scott JM (1978) The identification of the products of folate catabolism in the rat. Br J Haematol 38:211–218
Scott JM (2001) Methyltetrahydrofolate: the superior alternative to folic acid. In: Nutraceuticals in health and disease prevention. Marcel Dekker, New York, pp 75–90
Ng X, Lucock MD, Veysey M (2008) Physicochemical effect of pH and antioxidants on mono- and triglutamate forms of 5-methyltetrahydrofolate, and evaluation of vitamin stability in human gastric juice: Implications for folate bioavailability. Food Chem 106:200–210
Lucock MD, Priestnall M, Daskalakis I, Schorah CJ, Wild J, Levene MI (1995) Non-enzymatic degradation and salvage of dietary folate: physico-chemical factors likely to influence bioavailability. Biochem Mol Med 55:43–53
Giovannucci E (2004) Alcohol, one-carbon metabolism, and colorectal cancer: recent insights from molecular studies. J Nutr 134:2475S–2481S
Kim YI (2004) Folate and DNA methylation: a mechanistic link between folate deficiency and colorectal cancer? Cancer Epidemiol Biomarkers Prev 13:511–519
Fallon UB (2003) Colon cancer, folate and genetic status. Int J Epidemiol 32:67–70
Lucock MD, Yates ZR (2009) Folic acid fortification: a double-edged sword. Curr Opin Clin Nutr Metab Care 12:555–564
MRC Vitamin Study Group (1991) Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. Lancet 338:131–137
Smith DA, Kim YI, Refsum H (2008) Is folic acid good for everyone? Am J Clin Nutr 87:517–533
Ulrich CM, Potter JD (2007) Folate and cancer—timing is everything. JAMA 297:2408–2409
Lucock MD (1999) eBMJ; http://www.bmj.com/cgi/eletters/319/7202/92#EL1
Wright AJ, Dainty JR, Finglas PM (2007) Folic acid metabolism in human subjects revisited: potential implications for proposed mandatory folic acid fortification in the UK. Br J Nutr 98:667–675
Bailey SW, Ayling JE (2009) The extremely slow and variable activity of dihydrofolate reductase in human liver and its implications for high folic acid intake. Proc Natl Acad Sci USA 106:15424–15429
Pickell L, Brown K, Li D, Wang XL, Deng L, Wu Q, Selhub J, Luo L, Jerome-Majewska L, Rozen R (2010) High intake of folic acid disrupts embryonic development in mice. Birth Defects Res A Clin Mol Teratol 91:8–19
Offer T, Ames BN, Bailey SW, Sabens EA, Nozawa M, Ayling JE (2007) 5-Methyltetrahydrofolate inhibits photosensitization reactions and strand breaks in DNA. FASEB J 21:2101–2107
Van der Put NMJ, Steegers-Theunissen RPM, Frosst P, Trijbels FJ, Eskes TK, van den Heuvel LP, Mariman EC, den Heyer M, Rozen R, Blom HJ (1995) Mutated methylenetetrahydrofolate reductase as a risk factor for spina bifida. Lancet 346:1070
Van der Put NMJ, Van der Molen EF, Kluijtmans LAJ, Heil SG, Trijbels JM, Eskes TK, Van Oppenraaij-Emmerzaal D, Banerjee R, Blom HJ (1997) Sequence analysis of the coding region of human methionine synthase: Relevance to hyperhomocysteinaemia in NTD and vascular disease. Q J Med 90:511–517
Van der Put NM, Gabreels F, Stevens EM, Smeitink JA, Trijbels FJ, Eskes TK, van den Heuvel LP, Blom HJ (1998) A second common mutation in the methylenetetrahydrofolate reductase gene: an additional risk factor for neural tube defects? Am J Hum Genet 62:1044–1051
Johnson WG, Stenroos ES, Spychala JR, Chatkupt S, Ming SX, Buyske S (2004) New 19 bp deletion polymorphism in intron-1 of dihydrofolate reductase (DHFR): a risk factor for spina bifida acting in mothers during pregnancy? Am J Med Genet A 124:339–345
Winkelmayer WC, Eberle C, Sunder-Plassmann G, Fodinger M (2003) Effects of the glutamate carboxypeptidase II (GCP2 1561C>T) and reduced folate carrier (RFC1 80G>A) allelic variants on folate and total homocysteine levels in kidney transplant patients. Kidney Int 63:2280–2285
Wilson A, Platt R, Wu Q, Leclerc D, Christensen B, Yang H, Gravel RA, Rozen R (1999) A common variant in methionine synthase reductase combined with low cobalamin (vitamin B12) increases risk for spina bifida. Mol Genet Metab 67:317–323
Melse-Boonstra A, Lievers KJ, Blom HJ, Verhoef P (2004) Bioavailability of polyglutamyl folic acid relative to that of monoglutamyl folic acid in subjects with different genotypes of the glutamate carboxypeptidase II gene. Am J Clin Nutr 80:700–704
Rady PL, Szucs S, Grady J, Hudnall SD, Kellner LH, Nitowsky H, Tyring SK, Matalon RK (2002) Genetic polymorphisms of methylenetetrahydrofolate reductase (MTHFR) and methionine synthase reductase (MTRR) in ethnic populations in Texas; a report of a novel MTHFR polymorphic site, G1793A. Am J Med Genet 107:162–168
Miller JW, Ramos MI, Garrod MG, Flynn MA, Green R (2002) Transcobalamin II 775G>C polymorphism and indices of vitamin B12 status in healthy older adults. Blood 100:718–720
Pietrzyk JJ, Bik-Multanowski M (2003) 776C>G polymorphism of the transcobalamin II gene as a risk factor for spina bifida. Mol Genet Metab 80:364
Tsai MY, Bignell M, Schwichtenberg K, Hanson NQ (1996) High prevalence of a mutation in the cystathionine beta-synthase gene. Am J Hum Genet 59:1262–1267
Heil SG, Van der Put NM, Waas ET, den Heijer M, Trijbels FJ, Blom HJ (2001) Is mutated serine hydroxymethyltransferase (SHMT) involved in the etiology of neural tube defects? Mol Genet Metab 73:164–172
Horie N, Aiba H, Oguro K, Hojo H, Takeishi K (1995) Functional analysis and DNA polymorphism of the tandemly repeated sequences in the 5′-terminal regulatory region of the human gene for thymidylate synthase. Cell Struct Funct 20:191–197
Tan W, Miao X, Wang L, Yu C, Xiong P, Liang G, Sun T, Zhou Y, Zhang X, Li H, Lin D (2005) Significant increase in risk of gastroesophageal cancer is associated with interaction between promoter polymorphisms in thymidylate synthase and serum folate status. Carcinogenesis 26:1430–1435
Ulrich CM, Bigler J, Velicer CM, Greene EA, Farin FM, Potter JD (2000) Searching expressed sequence tag databases: discovery and confirmation of a common polymorphism in the thymidylate synthase gene. Cancer Epidemiol Biomarkers Prev 9:1381–1385
Mosharov E, Cranford MR, Banerjee R (2000) The quantitatively important relationship between homocysteine metabolism and glutathione synthesis by the transsulfuration pathway and its regulation by redox changes. Biochemistry 39:13005–13011
Lucock M (2004) Is folic acid the ultimate functional food component for disease prevention? Br Med J 328:211–214
Matthews RG, Haywood BJ (1979) Inhibition of pig liver methylenetetrahydrofolate reductase by dihydrofolate: some mechanistic and regulatory implications. Biochemistry 18:4845–4851
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lucock, M., Yates, Z., Boyd, L. et al. Vitamin C-related nutrient–nutrient and nutrient–gene interactions that modify folate status. Eur J Nutr 52, 569–582 (2013). https://doi.org/10.1007/s00394-012-0359-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-012-0359-8